Maintenance of genome stability in the face of DNA damage is essential for cellular homeostasis and prevention of cancer and brain degeneration. The DNA damage response (DDR) is a complex response that is rapidly activated when a DNA lesion occurs in chromosomal DNA. Mutations affecting the proteins involved in the DDR can lead to genomic instability syndromes that involve tissue degeneration, cancer predisposition, premature aging, and brain mal-development and degeneration. Mutation of the kinase ATM leads to a prototype genomic instability syndrome, ataxia-telangiectasia (A-T). A-T is characterized by progressive cerebellar degeneration, immunodeficiency, genome instability, premature aging, gonadal dysgenesis, extreme radiosensitivity, and high incidence of lymphoreticular malignancies. One of the most devastating symptoms of A-T — cerebellar ataxia — develops progressively into general motor dysfunction. Based on our previous studies we hypothesized that the neurological deficits in genomic instability disorders stem (at least in part) from significant reduction in functionality of glial cells. We further hypothesized that impaired vascularization affects the environment in which the neurons and glial cells function, thereby reducing neuronal cell functionality. We found that ATM deficiency led to aberrant astrocytic morphology and alterations of vasculature both in cerebellum and the visual system. Moreover, we found reduced myelin basic protein immunoreactivity and signs of inflammation in ATM-deficient cerebella and optic nerve. Interestingly, similar findings have been reported in patients with other genomic instability disorders. These observations bolster the notion that astrocyte-specific pathologies and hampered vascularization and astrocyte-neuron interactions in the CNS play crucial roles in the etiology of genome instability brain disorders and underlie brain degeneration at specific sites.
Introduction
Although brain degenerative diseases (BDDs) have been thought to be primarily associated with dysfunction or death of neural cells, emerging evidence indicates that dysfunction in neuron-glia-vascular communications play a much larger role than was appreciated. Indeed, a growing body of evidence supports the idea that glial cells, and astrocytes in particular, are much more than just the “glue” that holds together the neurons of the brain. Rather, glia are now seen as very active participants in the neuronal network. Glia can release “gliotransmitters” and have many of the same receptors as neurons, indicating that these cells can speak and comprehend the chemical language of neurons. Accordingly, it no longer makes sense to consider networks of neurons in isolation, since neurons and glia act in concert to form the circuits and networks of the brain. Given the importance of neuron-glia-vascular communications and the insights stemming from a large body of previous work, we hypothesize that dysfunctional neuron-glia-vascular communications play a major role in BDDs in general and in ataxia-telangiectasia (A-T) in particular.
The DNA damage response and the ATM protein
An aberrant response to DNA lesions is implicated in many human brain degenerative disorders (Abner and McKinnon, 2004; Barzilai et al., 2008). In healthy cells, DNA damage is rapidly detected, leading to activation of an intricate web of signalling pathways known as the DNA damage response (DDR). On the other hand, in cells with brain degenerative dysfunction, activities of some components of the DDR machinery are impaired (Barzilai et al., 2008) . Under normal conditions this response culminates in activation of cell-cycle checkpoints and appropriate DNA repair pathways and, in certain contexts, in initiation of apoptotic programs.
The DNA damage response is a hierarchical process executed through a series of steps. The DNA lesions are detected by sensor proteins that recognize the lesions themselves or chromatin alterations that result from the DNA damage. Transducers are then brought into action to convey the damage signal to downstream effectors. It is this relay system from transducers to effectors that enables a single DNA lesion to modulate numerous pathways. The transducers might also be involved in the assembly of DNA-repair complexes at the sites of DNA damage (reviewed in (Iliakis et al., 2003; Shiloh, 2003; Su, 2006; Zhou and Elledge, 2000) ). In particular, following sensing of a DNA double-strand break (DSB), ATM protein is activated and a portion of nuclear ATM binds to DSB sites (Andegeko et al., 2001; Meyn, 2003) . Activation of ATM involves autophosphorylation of serine 1981 and subsequent dissociation of inactive ATM dimers into active monomers (Bakkenist and Kastan, 2003). The fraction of ATM that binds to DNA is also autophosphorylated (Uziel et al., 2003) , but recent data indicate that this autophosphorylation is not necessary for ATM recruitment to damage sites (Meyn, 2003) . Activation of the ATM kinase seems to be an initiating event in cellular responses to irradiation. ATM may be activated by other stressors in addition to DSBs (Kurz and Lees-Miller, 2004) . Downstream of the transducer proteins are targets that control various cellular processes such as DNA repair, cell cycle progression, gene transcription, protein synthesis, aging and degradation, and apoptosis.
Mutations in key DDR molecules are associated with human genomic instability syndromes (Shiloh, 2003) . These disorders include, for example, A-T (mutation in ATM) (Shiloh, 2014) , Ataxia-Telangiectasia Like Disorder (A-TLD, mutated in MRE11) (Taylor et al., 2004) , Seckel Syndrome (mutated in ATR) (O'Driscoll et al., 2004) , and Nijmegen Breakage Syndrome (NBS, mutated in NBS1) (Chrzanowska et al., 2012) . The DDR pathway is depicted schematically in Figure 1.
A-T and associated genomic instability disorders
The ATM protein is the product of the ATM gene. ATM-null mutations cause the autosomal recessive disorder, A-T (Savitsky et al., 1995a; Savitsky et al., 1997; Savitsky et al., 1995b) . A-T is characterized by progressive cerebellar degeneration, immunodeficiency, genome instability, premature aging in some patients, gonadal dysgenesis, extreme radiosensitivity, and high incidence of lymphoreticular malignancies (Biton et al., 2008; Lavin and Shiloh, 1997) . One of the most devastating symptoms of A-T — cerebellar ataxia — develops progressively into general motor dysfunction (Crawford, 1998) . Major causes of death in A-T patients are malignancies or aspiration due to cerebellar-related swallowing difficulties. Post-mortem studies revealed a significant loss of Purkinje and granule neurons in the cerebellum of children with A-T, and therefore A-T was once considered a “Purkinje cell disease”. Clearly, cerebellar neurons are seriously damaged due to the loss of ATM. Patients suffering from A-TLD and NBS syndromes exhibit symptoms involving neural and lymphoid organs. The phenotype of A-TLD is similar to that of A-T with a slower progression; NBS patients exhibit the cellular phenotype of A-T but do not have cerebellar defects (Digweed and Sperling, 2004; Lavin, 2008; Stracker and Petrini, 2011) . The phenotype of the Seckel syndrome overlaps with some features of A-T, A-TLD, and NBS including microcephaly, mental retardation, genomic instability, and hematological malignancies. Among all these diseases, neurological defects are common, suggesting that the DNA damage response controls neurogenesis and neurodegeneration.
Most, if not all, A-T research on the neurological aspects of the disease has focused solely on Purkinje cells; for many years other types of neuronal cells and especially glial cells were not considered to play a role in the disease (Barzilai et al., 2008; Eilam et al., 2003; McKinnon, 2009, 2012) . It has now become evident that astroglial cells are as diverse as neurons; they shape the micro-architecture of the brain matter; they express the same receptors and channels as neurons do; they receive synaptic inputs; they are able to release ‘glio’ transmitters and produce long-range information exchange; and finally, they act as pluripotent neural stem cells for adult neurogenesis (Haydon and Carmignoto, 2006; Volterra and Meldolesi, 2005) . Despite this, the role of glia in the progression and handling of insults to the nervous system has been largely neglected. Indeed, brain pathology, is, to a very great extent, a pathology of glia.
Mouse models of A-T
Important tools in the investigation of the physiological and molecular bases of A-T are mouse models obtained using gene targeting or transgene expression. ATM-deficient mice exhibit many of the characteristics of human A-T patients (Barlow et al., 1996; Elson et al., 1996; Hande et al., 2001; Xu et al., 1996) . The most cardinal feature of A-T - neuronal degeneration and the associated neuromotor dysfunction - are very mild and highly variable in these mice, however (Barlow et al., 1996; Borghesani et al., 2000; Elson et al., 1996; Xu et al., 1996) . A simple explanation for the intriguing lack of overt cerebellar degeneration in ATM-deficient mice could be that to obtain this phenotype in the mouse may require suppression of the DDR beyond that achieved by eliminating ATM.
The role of malfunctioning vasculature in neurodegenerative diseases
Some brain disorders may have a vascular origin (Baldwin and O'Brien, 2002; Forstl and Howard, 1991) , and vascular diseases can be directly linked to neuronal and synaptic dysfunction through changes in the blood flow that increase blood-brain barrier (BBB) permeability and nutrient supply (Zlokovic, 2008) . As the brain lacks a reserve of glucose and oxygen, it is wholly dependent on a constant blood supply. A threat to cerebral perfusion is likely to have dramatic consequences on neuronal functions. Vascular diseases may lead to activation of astrocytes and microglial cells resulting in elevated expressions of inducible nitric oxide synthase (iNOS) and release of neurotoxic reactive oxygen species and nitric oxide. The inflammatory mechanisms may be aggravated by continuous release of chemokines such as CCL2 and CCL3 by astrocytes (McKimmie and Graham, 2010) . Furthermore, oxidative stress and glucose starvation may lead to the impairment of astrocyte glutamate uptake, which in turn results in glutamate neurotoxicity.
Here we show that malfunctioning DDR can lead to progressive degeneration of certain CNS sub-organs such as the cerebellum and the visual system. In addition to neuronal demise, malfunctioning DDR severely affects the vascular system and glia cells and stimulates brain inflammation. It is our notion that the common denominator of brain degeneration induced by malfunctioning DDR stems from reduced functionality of the glial cells.
Figure 1. Schematic depiction of the DDR machinery following activation by DSBs
Materials and Methods
Animals
ATM–/– mice (Barlow et al., 1996) were a generous gift from Dr. Anthony Wynshaw-Boris (University of California, San Diego, CA, USA). Offspring of these mice were genotyped using PCR-based assays based on mouse-tail DNA prepared using the GenElute Mammalian Genomic DNA Miniprep kit (Sigma, St. Louis, MO, USA). ATM–/– mice at different ages were used for this study, and age-matched ATM–/– littermates (WT) were used as controls. Mice were housed and maintained in the animal facility of Tel Aviv University, and all experiments complied with protocols approved by the university’s animal care committee.
Immunohistochemical analysis of cerebellar and optic nerve sections
Sections were fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 10 min, were washed in PBS for 10 min, and then incubated with blocking solution containing 1% bovine serum albumin (BSA) (Sigma) and 10% normal donkey serum (Jackson ImmunoResearch, Baltimore, MD, USA) in PBS for 1 h at room temperature. The sections were incubated overnight with primary antibody in 0.25% Triton X-100 at 4 °C. The slides were washed three times with PBS and incubated with the appropriate secondary antibody: α-calbindin antibody (1:1,000, Sigma), rabbit α- glial fibrillary acidic protein (GFAP; 1:200, Sigma), α-VEGF (1:1000; Abcam, Cambridge, UK), or α-IBA1 (Wako Chemicals, Richmond VA) and incubated for 1 h at room temperature. After one wash with PBS and two in a buffer containing 10 mM Tris and 1 mM ethylene diamine tetra acetic acid, the sections were incubated with the nucleic acid dye Sytox blue (Molecular Probes, Invitrogen, Carlsbad, Germany) for 30 min, slides were then washed three times with the same buffer and mounted with aqueous mounting medium containing anti-fading agents and 4′-6-diamidino-2-phenylindole (DAPI) for nuclei marker (VECTOR, Burlingame, CA, USA). Observations and photography were carried out using a Zeiss LSM 510 META confocal microscope (Oberkochen, Germany).
Immunoblotting analysis
Tissues were washed with ice-cold PBS and homogenized in ice-cold homogenization buffer [150 mM NaCl, 10 mM Tris, pH 7.6, 1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS, 1:50 phosphatase inhibitor cocktails I and II (Sigma), and 1:100 protease inhibitor cocktail (Sigma)]. Protein concentration was determined using a Bradford assay (Bradford, 1976) with BSA as a standard. Blots were prepared as described by Harlow and Lane (Harlow, 1988) using 10% polyacrylamide gels. Each lane was loaded with 50 μg of protein extract, and after the electrophoresis, the proteins were transferred to an Immobilon polyvinylidiene disulfide membrane (Millipore, Billerica, MA) for at least 12 h at 220 mA. The vascular endothelial growth factor (VEGF) antibody (1:1000; Abcam) was used. Secondary antibody was goat anti-rabbit, IRDye-conjugated (Li-Cor antibody, 1:1000; Li-Cor Biosciences, Lincoln, NE, USA) for 1 h at room temperature. Blots were scanned using the Li-Cor imaging system, and band intensity was analysed using Odyssey software (Odyssey Software, West Henrietta, NY, USA).
Quantitative real-time PCR
Samples were analysed for quantification of mRNA expression via reverse transcription followed by quantitative real-time polymerase chain reaction using TaqMan (Absolute Blue QPCR ROX Mix, AB-4138; Abgene). RT-PCR assays were designed by Applied Biosystems (Foster City, CA, USA), and b-actin RNA was used for normalization (Frenkel et al., 2008). The comparative Ct method was used for quantification of transcripts according to the manufacturer’s protocol. Measurement of DCt was performed in duplicate or triplicate. All reactions were performed with primer concentrations of 0.25 mmol/L in a total volume of 20 mL.
Angiography
Mice were anesthetized by an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (4 mg/kg). An incision was made into the peritoneal cavity, and the diaphragm and pericardium were incised to visualize the heart. The mice were perfused slowly through the left ventricle with saline containing 1% albumin-conjugated FITC (Sigma). Retinal arteries were labelled with Alexa 633 (Invitrogen). Mice were sacrificed by CO2 inhalation; the eyes were enucleated and fixed in 4% paraformaldehyde for 1 h. The cerebella were isolated, clarified, and 400-mm sections were prepared. The retinas were gently separated from the eyecups and mounted on a microscope slide with mounting medium. Four incisions were made to flatten the retina, which was then covered with a coverslip. Imaging was performed on a fluorescence microscope (Zeiss LSM 510).
Statistical analysis
Data from each experiment are expressed as means ± standard errors of the mean (SEM). Two-tailed Student’s t tests were performed when two groups were compared. The one-way ANOVA followed by Bonferroni’s multiple comparison tests was used for multiple samples. Statistical significance was determined at P<0.05.
Results
Signs of vascular impairments in ATM−/− cerebellar folia
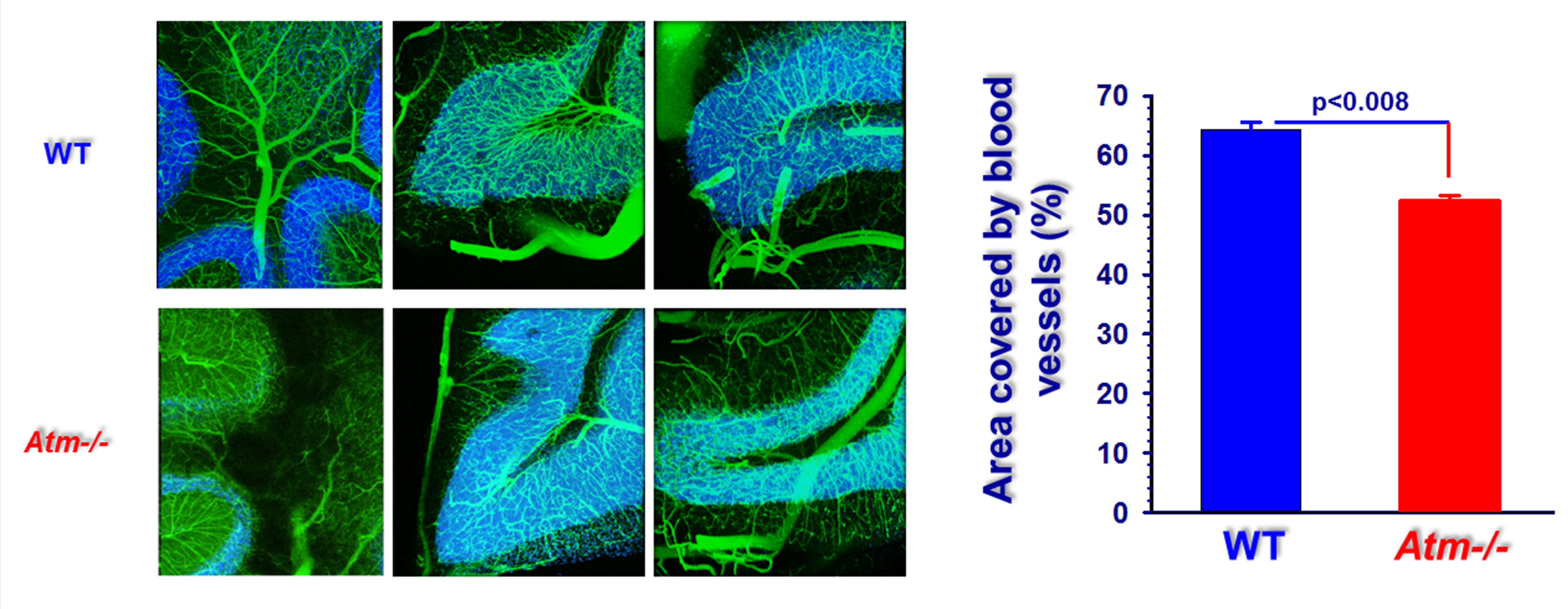
The effects of ATM deficiency on the mouse cerebellar vascular system were apparent in analysis focused on a cerebellar folia, which contains Purkinje, granule, basket, and satellite neurons as well as glial cells and rich vascular network (Figure 2). ATM deficiency clearly resulted in cerebellar vascular impairment as evident from the morphology of the mutant blood vessels as well as in high background indicative of leaky blood vessels in ATM−/− brains compared to those from WT animals. These results are similar to our previously published results (Raz-Prag et al., 2011). Quantitative analysis revealed that the area covered by blood vessels in cerebellar folia is significantly smaller in ATM-deficient mice.
Figure 2. High-throughput volumetric angiographs derived from WT and ATM−/− cerebella. Maximum intensity projection over 3.4 mm2 and across 500 μm in depth of cerebellar vasculature. Tiles of 400x400x500 μm were obtained by automated two-photon laser scanning microscopy of a sagittal section of a mouse cerebellum. A low-viscosity fluorescent gel was perfused transcardially to highlight the vasculature. Data were acquired at sampling resolution of 1 pixel/μm
Arterial impairment in ATM−/− retina
In our previous study, we found that ATM deficiency severely affects the integrity of the retinal vascular system but were unable to distinguish between veins and arteries (Raz-Prag et al., 2011). In the current study we labelled both the total blood vessels using albumin-conjugated FITC and selectively labelled the arteries with Alexa 633. Blood vessels in the retinas of ATM−/− mice were labelled faintly compared to the WT retinas. Vessels in the ATM−/− retinas appear constricted relative to those in WT (Figure 3), and our data suggest that the vessel density is lower in ATM−/− retinas. Moreover, ATM deficiency resulted in leaky arteries with microhaemorrhages in the vicinity of ATM−/− arteries but not near WT arteries (Figure 3).
Increased expression of VEGF in ATM−/− retinas
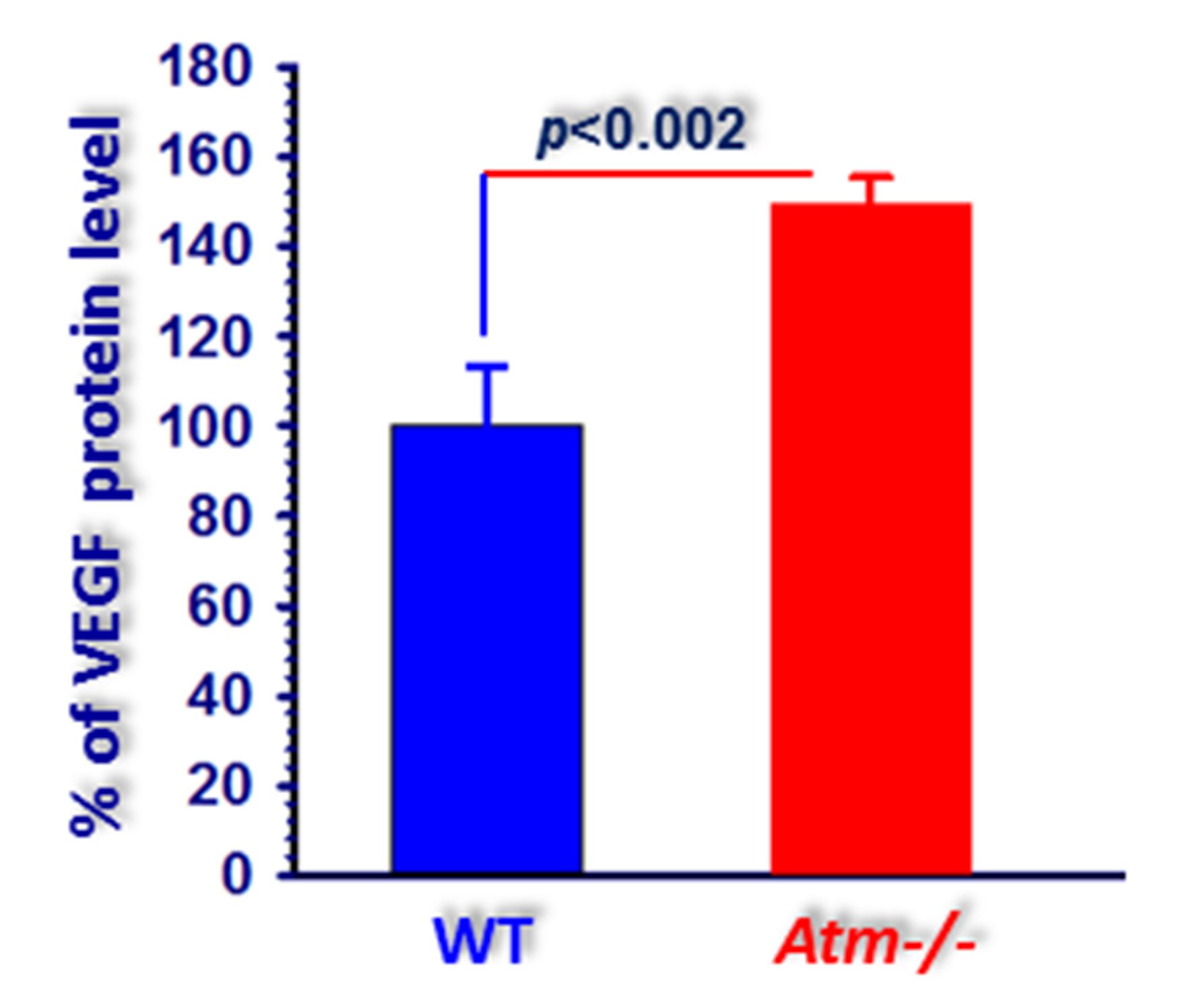
To further analyze the effects of ATM deficiency on astrocyte-associated factors, we measured the levels of VEGF in the cerebellum. It has been shown that VEGF plays a major role in angiogenesis, and under pathological conditions, changes in VEGF levels can cause vascular dysfunction (Detmar et al., 1997; Shweiki et al., 1992). Furthermore, it was suggested that VEGF may alter vascular permeability by regulating tight junctions (Harhaj and Antonetti, 2004). Therefore, we expanded our analysis and asked whether the vascular differences we observed between cerebella and retinas of ATM−/− and WT mice are correlated with changes in VEGF expression levels. As shown in Figure 4, significantly higher levels of VEGF protein were measured in the cerebellum samples fromATM−/− compared to WT mice. These findings are similar to the results of our previous study in which the VEGF levels were found to be significantly higher in ATM-deficient retina (Raz-Prag et al., 2011).
Figure 3. Attenuated blood vessels in retinas of ATM−/− mice. Flat-mount retinas of 2-month-old WT (left panel) and ATM−/− (right panel) mice imaged with a fluorescent microscope following intracardial perfusion with albumin-conjugated FITC albumin-conjugated FITC and Alexa 633 to selectively labelled the arteries. Constricted arteries are indicated by green arrows, and microhaemorrhages are indicated by yellow arrows
Alterations in glial cell functionality in ATM-deficient mice
Neurotrophic factors play an important role in the maintenance of the CNS homeostasis. Astrocytes are known to produce and secrete their own repertoire of neurotrophic factors including BDNF and NT3 (Ma et al., 2005). Recent data suggest that a dysfunction in glial cell activity may contribute to the pathogenic process that leads to brain degenerative diseases (Ma et al., 2005). In our previous study using a Nijmegen breakage syndrome mouse model we found that astrocyte dysfunction is associated with cerebellar attrition and that less microglial recruitment occurs in the NBS1-D-CNS brains than in WT brains (Galron et al., 2011). There is also a significant reduction in the secretion of neurotrophic factors, such as BDNF and NT3 in the mutant brains. The functionality of the NBS1-mutant astrocytes is severely reduced as evidenced by the decreased expression of glutamine synthetase, which converts glutamate to glutamine. Reduced levels of BDNF and NT3 are also found in ATM−/− cerebella (Meshulam et al., 2012).
Staining of astrocytes with GFAP revealed a less complex cell arborisation in ATM−/− versus WT cultures (Fig. 5), and the number of branches originating from the cell bodies was significantly lower in ATM−/− astrocytes (average value 3.65 ± 1; n=12, 3 different cultures) than in WT cells (7.5 ± 1.5; n=12, 3 different cultures; p<4.7x10-7, two-tailed t test). To test whether astrocytic morphological alterations are restricted to dissociated cell cultures or also appeared in in vivo, we stained cerebellar sections as well as retinas and optic nerves with GFAP. A marked reduction in cerebellar GFAP staining was seen in ATM-deficient mice compared to WT (Fig. 5) suggesting that ATM deficiency leads to reduced levels of velate astrocytes. Similarly, morphological alterations in astrocytes were also found in ATM-deficient optic nerves. Whereas in WT optic nerve the astrocytes are almost evenly spread in the axons, markedly higher levels of astrocytes were detected on both sides of the optic nerve in the ATM-null samples (Fig. 6). Reduced arborisation of the retina and optic nerve were associated with reduced retinal functionality as evidenced electroretinographic examination reported in our previous study (Raz-Prag et al., 2011) suggesting that impaired astrocytic functionality in the central nervous system plays an important role in the aetiology of A-T.
Figure 4. Increased VEGF levels in ATM−/− cerebella. Calculations based on western blot analyses reveal significantly higher levels of VEGF in eyecups of ATM−/− mice relative to VEGF levels in WT control mice. Statistical values were calculated by 2-tailed Student’s t-test
Signs of inflammation in ATM-deficient cerebellum
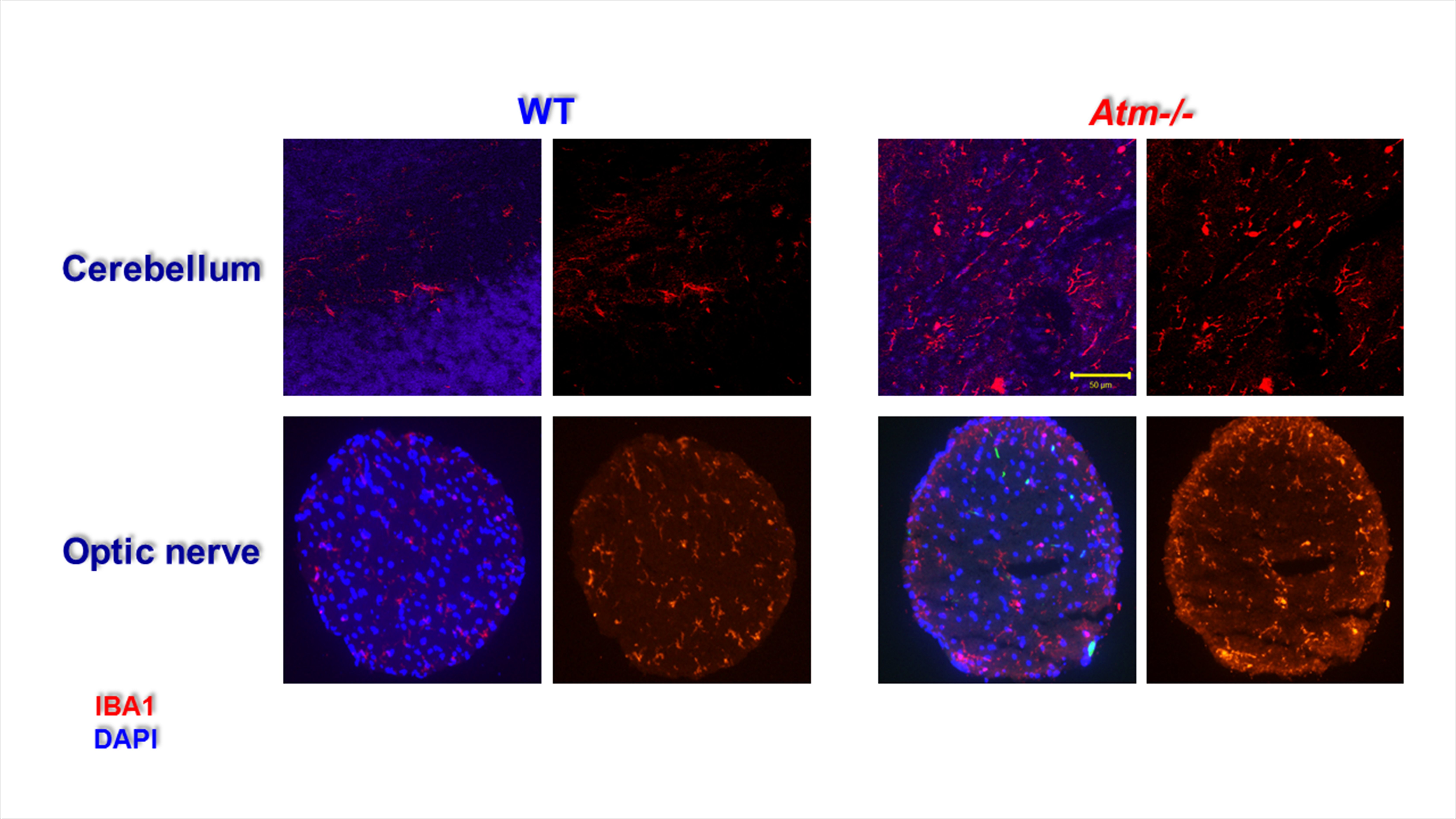
Inflammation is thought to be associated with many BDDs such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, and multiple sclerosis (MS). To test whether malfunctioning DDR can lead to inflammation we evaluated expression of IBA1, which is a marker for macrophages and microglia. Higher levels of macrophages were found in ATM-deficient cerebellum than in WT samples indicating that ATM deficiency leads to chronic inflammation in various part of the CNS (Fig. 7, upper panel). Moreover, the morphology of the IBA1-positive cells was suggestive of an activated state: There were more processes in the IBA1-positive cells in the ATM-deficient cerebellum than in WT cerebellum. Similarly, higher levels of IBA1-positive cells were detected in ATM-deficient optic nerves than in the controls (Fig. 7, lower panel). Interestingly, in ATM−/− retinas, most of macrophages were located at the edge of the axons suggesting that the increased levels of immune cells result from cell invasion rather than proliferation.
Figure 5. Astroglial cell alterations in primary cultures and CNS sections derived from WT and ATM−/− cerebella. Zoom on cultured single astrocyte morphology (left panels) and astrocytic morphology in vivo (middle panels) highlights a reduced number of branches originating from the cell body in the ATM−/− compared to WT samples. Right panels: Numbers of velate astrocytes and Bergmann glia are reduced in 4 and 14 month-old mice ATM−/− cerebellar sections compared to WT
Reduced expression of myelin basic protein (MBP) in ATM–/– and optic nerve
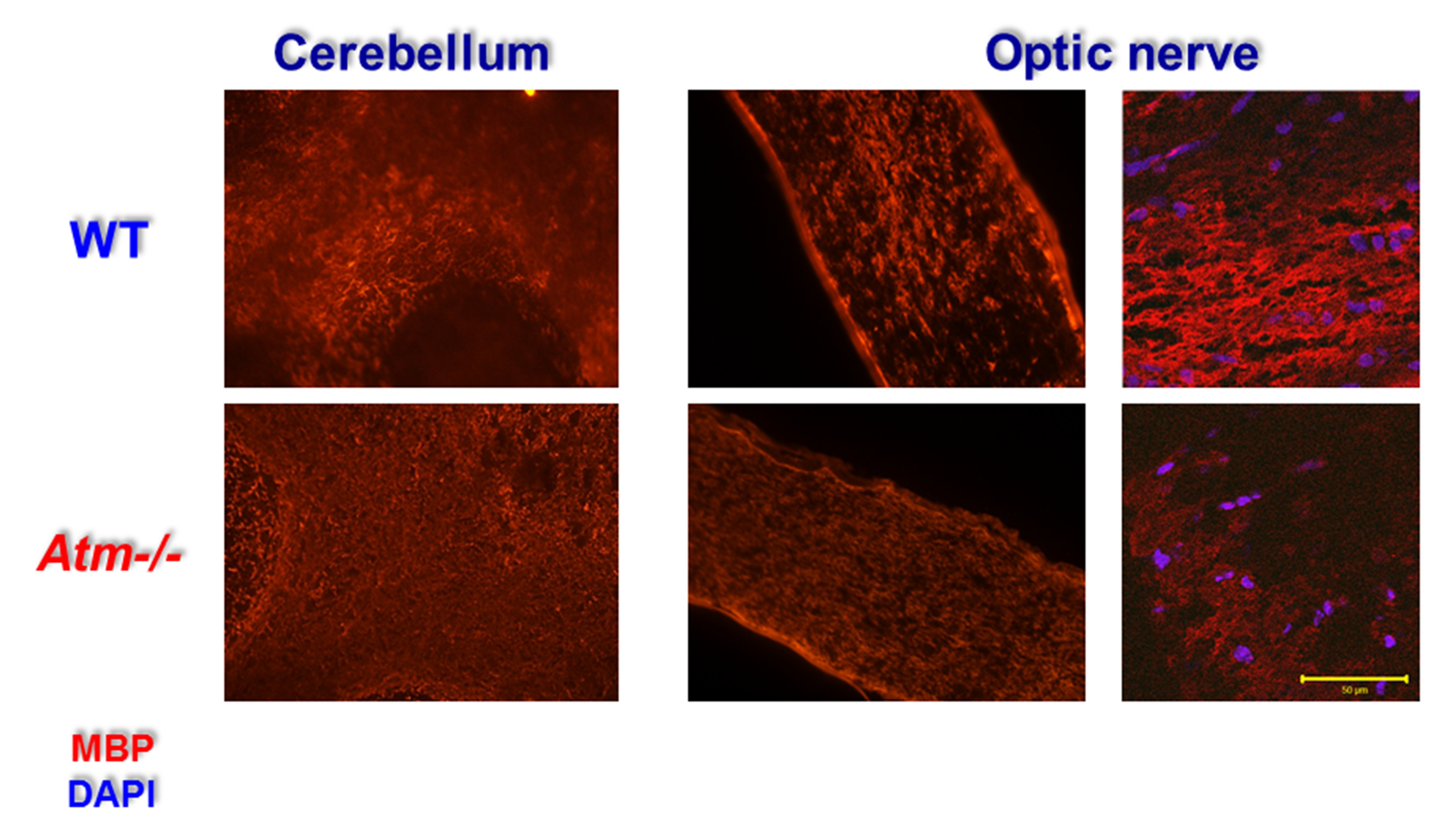
In a previous study (Assaf et al., 2008), we found that specific ablation of NBS1 in the CNS led to myelin loss in cerebellar tissue and in optic nerve. Whereas in WT cerebellar section one can easily see the myelin that enwraps axons, very few myelinated axons were observed in ATM-deficient cerebellar sections (Fig. 8). The myelin loss was even more evident in the optic nerve of ATM–/– mice. Clear myelinated axons are detected in WT optic nerve but almost none in the mutant optic nerve (Fig. 8).
Figure 6. Astroglial cell alterations in ATM-deficient optic nerve and retina. Alterations in astrocyte morphology were evident in ATM−/− optic nerve (left and middle panels). (Right Panel). Whereas in WT retinas the astrocytes are spread evenly in the optic nerve, in the ATM−/− optic nerve the astrocytes are concentrated at the walls of the optic nerve
Discussion
This study clearly shows that ATM depletion resulted in marked impairment in cerebellar and retinal vascularization as well as in morphological alterations in astrocytic cells. In addition, ATM deficiency was associated with increased inflammation and demyelination in both cerebellum and optic nerve. Interestingly, these findings resemble the CNS pathology that was observed in Nijmegen breakage syndrome, which is an A-T related disorder (Galron et al., 2011) . Both ATM and NBS1 are important components of the DDR machinery. The fact that similar impairments are induced upon loss of either protein suggests that malfunctioning DDR is responsible for these effects.
The current dogma in the field is that ATM deficiency in mice does not lead to cerebellar pathology. However, our study clearly showed that aged ATM−/− mice (> 1 year) develop progressive cerebellar degeneration (Bihari et al unpublished). These results suggest that if given sufficient time, ATM−/− mice will develop cerebellar atrophy that recapitulates the human A-T phenotype thereby providing a satisfactory animal model of A-T. Thus, it is fully justified to use this model system to study the role of ATM deficiency in brain pathology. Our study showed that the number of astrocytes are significantly reduced in ATM−/− mice compared to WT animals and that astrocytic arborisation was also decreased as previously observed (Meshulam et al., 2012) . These findings strengthen our hypothesis that astrocytes are key players in the etiology of A-T. In the past, astrocytes have traditionally been considered as passive elements and satellite cells of the CNS, which provide metabolic support for neurons and regulate extracellular homeostasis. Evidence accumulated in the last decade indicates that astrocytes are also actively involved in the control of neuronal functions, communication pathways, and plasticity and are able to receive signals from neurons and release neuroactive substances (Kirchhoff et al., 2001; Ricci et al., 2009) including signalling of glutamatergic (Nagler et al., 2001; Ullian et al., 2001) and GABAergic synapses (Hughes et al., 2010) .
Figure 7. Increased IBA1-positive cells in ATM-deficient CNS tissues. The cerebella isolated from 4-month-old WT and ATM−/− mice were fixed, longitudinally transacted, and immnuoreacted with IBA1, a marker of macrophages. Markedly higher levels of IBA1 were detected in ATM−/− optic nerves indicative of chronic inflammation
A-T is an early onset genomic instability disorder (Lavin and Shiloh, 1997; Shiloh, 1995). Patients also displays signs of premature aging (Barzilai et al., 2016) and progressive cerebellar pathology (Crawford, 1998; Crawford et al., 2000; Crawford et al., 2006). These observations suggest that A-T has developmental and progressive branches. Our work provides evidence for early alterations in astrocytes as demonstrated by reduced arborisation in cultured ATM−/− astrocytes derived from newborn mice compared to those cultured from WT mice (Meshulam et al., 2012). Since neural migration during cerebellar development (Rakic, 1971) is highly dependent on glial cells, it is possible that malfunctioning astrocytes hamper neuronal migration into the cerebellum and resulting in abnormal development and organization. It is also plausible that in A-T, in addition to migration, the astrocytes and the neurons never reach full compatibility leading to conditions that do not enable the neurons to thrive due to lack of proper support of the newly migrated neurons. Post-mortem analyses of young A-T patients support this notion; however, this is not seen in young mice (Paula-Barbosa et al., 1983).
A-T has also a progressive component. It is conceivable that during the generation and progression of certain brain degenerative disorders such as A-T, astrocytes that were compatible with the neurons undergo changes that impair their ability to maintain this compatibility. This situation describes the phenomenon known as “non-cell-autonomous effect” in which genotypically mutant cells cause other cells (regardless of their genotype) to exhibit a mutant phenotype. Indeed, studies over the last decade support the notion that the cellular basis of brain degenerative disorders is not simply cell autonomous. An important example of non-cell autonomous effects is observed in patients with amyotrophic lateral sclerosis (ALS), which is a degenerative disease characterized by motor neuron death. ALS can be induced by mutations in the gene encoding superoxide dismutase 1 (SOD1). Evidence for the non-cell-autonomous nature of ALS emerged from the observation that WT glial cells extend the survival of SOD1-mutant motor neurons in chimeric mice (Marchetto et al., 2008).
This led to the suggestion that astrocyte replacement might be a promising therapy for various brain degenerative diseases (Kruminis-Kaszkiel et al., 2014). Moreover, Han et al, have shown that the engraftment of human glial progenitor cells (GPCs) into neonatal immunodeficient mice led to their maturation into astrocytes with all the characteristics of human astrocytes. Remarkably, the chimeric mice displayed enhanced long-term potentiation and an enhanced ability to learn compared to the immunodeficient controls (Han et al., 2013). This study clearly showed that engrafted glia maintain their intrinsic ability to properly differentiate into human astrocytes in the mouse and enhance both activity-dependent plasticity and learning. Our data, in conjunction with other findings, indicate the importance of glial cells in the etiology of many brain degenerative diseases. Glial cells are not only essential for maintaining a healthy well-functioning brain, but they also protect the brain and enhance the functional recovery from injuries. Moreover, glial cells play an essential role in synaptic transmission, thereby modulating neuronal activity (Barzilai, 2011, 2013; Giaume et al., 2007; Sofroniew and Vinters, 2010).
Figure 8. Reduced MBP expression in ATM-deficient cerebellum and optic nerve. WT and ATM–/– cerebellar (left panels) and sagittal optic nerve sections (middle and right panels) were immunoreacted with anti-MBP antibody. The sections were visualized either using regular or confocal fluorescent microscopy
The interactions between astrocytes and endothelial cells are crucial for the formation of the BBB and in vascular disease pathology. The BBB is a separation of circulating blood from the brain extracellular fluid in the CNS. It occurs along all capillaries and consists of tight junctions around the capillaries. Endothelial cells restrict the diffusion of microscopic objects (e.g., bacteria) and large or hydrophilic molecules into the cerebrospinal fluid, while allowing the diffusion of small hydrophobic molecules (O2, CO2, hormones). Cells of the BBB actively transport metabolic products, such as glucose, and specific proteins into the brain. Astrocyte cell projections called astrocytic feet (also known as glia limitans) surround the endothelial cells of the BBB, providing biochemical support to those cells. The BBB is distinct from the quite similar barrier between blood and cerebrospinal fluid, which is a function of the choroidal cells of the choroid plexus, and from the blood–retinal barrier (Ueno et al., 2016).
Our findings clearly show that ATM deficiency leads to impaired vasculature. One of the culprits of CNS vascular impairment could be VEGF. In the eye, excessive VEGF has been implicated in neovascularization and vascular leakage that occurs during age-related macular degeneration and in proliferative retinopathies (Aiello et al., 1994). VEGF is up-regulated in response to and may be causative of hypoxia and non-perfusion (Tolentino et al., 1996). In addition, VEGF can induce hypertrophy of endothelial cells of retinal capillaries at the expense of lumen diameter (Hofman et al., 2001; Tolentino et al., 1996). There was an increase in VEGF in the retinas as well as cerebella of ATM−/− mice compared to controls. This overexpression of VEGF may cause the vasoconstriction and vascular leakage that we observed in the retinas and cerebella of ATM−/− mice. This notion is further supported by morphological deterioration of the retinal arteries of the ATM−/− mice. Increased retinal (Raz-Prag et al., 2011) as well as cerebellar (Meshulam et al., 2012) fibrinogen observed in previous studies of ATM-deficient mice is most likely caused by leakage of blood vessels. ATM-dependent retinal blood vessel deterioration appears to be progressive. Whereas a marked decline retinal vasculature was detected in in ATM-deficient mice at the age of 4 months in here and in our previous study (Raz-Prag et al., 2011), Okuno et al. did not find any vasculature abnormalities in one-month-old ATM−/− mice (Okuno et al., 2012). Moreover, retinal electrical activity as measured using electroretinogram shows an age-dependent decline (Raz-Prag et al., 2011).
We also found that ATM deficiency led to reduced MBP immunoreactivity both in the cerebellum and the optic nerve. Severe myelin deficiency was also detected in the mouse model of NBS (Assaf et al., 2008). Similar to mouse models of genomic instability disorders, myelin abnormalities were also found in 32-year-old A-T patients (Amromin et al., 1979) and in a 17-year-old A-T patient (Barbieri et al., 1986). Taken together, these results suggest that myelin is highly vulnerable to malfunctioning DDR both in humans and mice. We suggest that failing ATM-deficient astrocytes cause myelin abnormalities induced by malfunctioning DDR. Astrocytes promote inflammation and demyelination, and activated astrocytes can form glial scars that prevent remyelination (Claycomb et al., 2013). Astrocytes can also function as mediators of myelination by promoting the proliferation of oligodendrocyte progenitor cells (Moore and Cao, 2008). Since ATM-deficient astrocytes are stressed they may result in inflammation that indirectly reduces myelination or may reduce myelination directly by affecting the levels and the functionality of oligodendrocytes. In MS patients, the degree of myelin damage is correlated with the degree of inflammation (DeLuca et al., 2006; Herz et al., 2010). In both A-T and MS, malfunctioning DNA damage repair and increased oxidative stress are observed (Lu et al., 2000; Satoh et al., 2005). Interestingly, microglia and activated astrocytes are hypothesized to have a critical role in the progression of MS (Correale and Farez, 2015; Diestel et al., 2003). Here we suggest that A-T might be at least partly an autoimmune disease; if this is the case, the immune response should be considered during patient treatment.
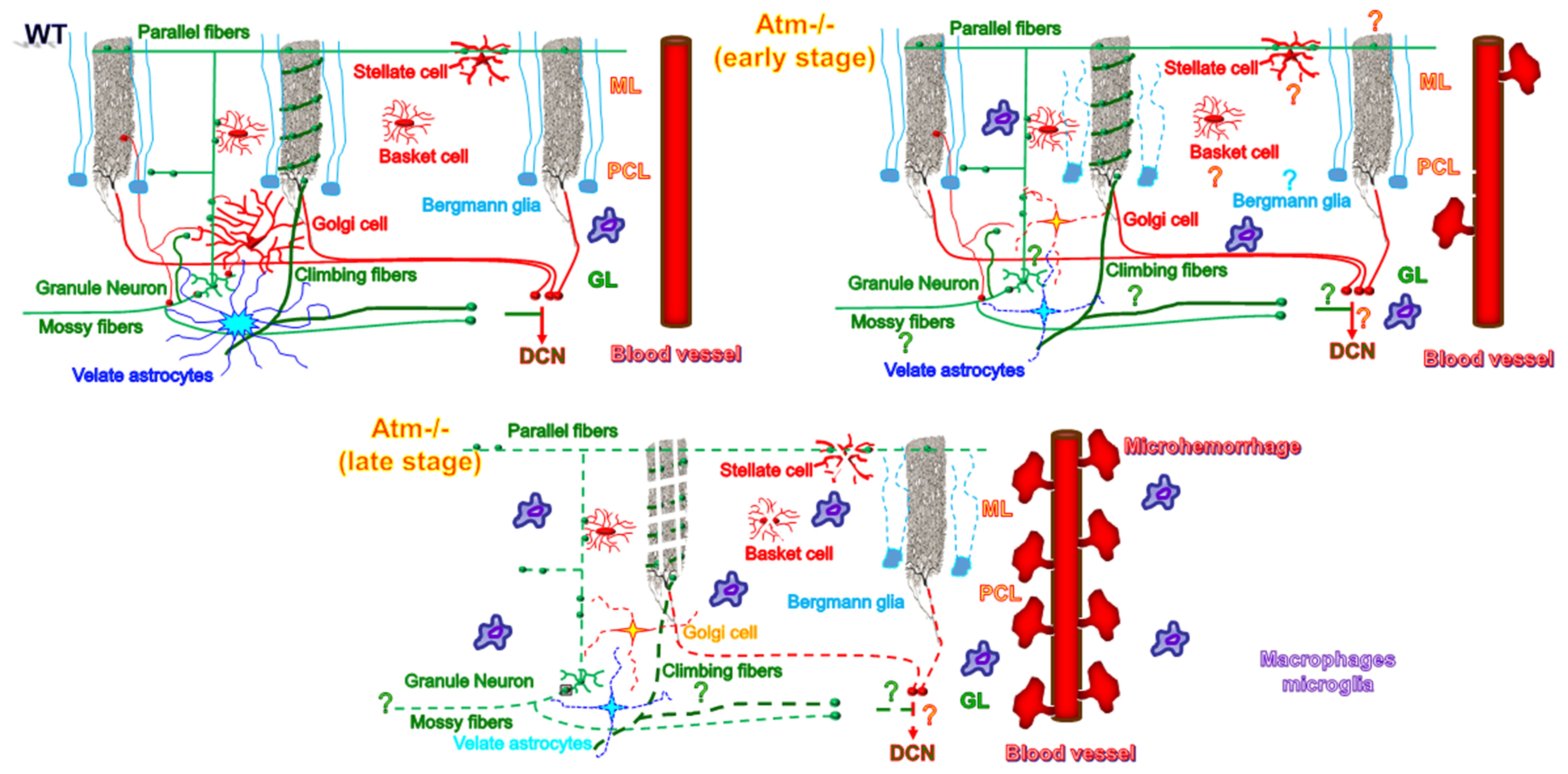
A-T is a glial-associated disease as shown schematically in Figure 9. Signs of glial stress can be detected at a very early age in ATM-deficient mice. With the progression of the disease we found further damage to astrocytes and Bergmann glial cells as well as increased levels of cerebellar and retinal microhaemorrhage. We also observed degeneration of Purkinje cell axons and condensation of the nuclei of the cerebellar granule neurons (unpublished data). Moreover, we found signs of chronic inflammation and reduced myelination in ATM-deficient cerebella.
In summary, until very recently, research into the mechanisms underlying brain degenerative diseases focused mainly on neuronal cells, their survival, their activity, their interactions, and their functions in the dynamics of neural circuits. Glial cells were long considered as merely as supportive cells. Accumulative data (Fellin et al., 2004; Fields et al., 2014; Halassa and Haydon, 2010) and the results shown here indicate that glial cells — and especially astrocytes — are key players in brain functionality. The importance of astrocytes and evidence from chimeric cultures and mice suggest that astrocyte transplants may be useful in future A-T treatments.
Figure 9. Cellular alteration in ATM-deficient cerebella. This model depicts the alterations that occur in early and in late stages of A-T. We suggest that astrocytes play a major role in the etiology of A-T. Malfunctioning astrocytes can lead to the demise of Purkinje as well as other types of cerebellar cells such as the inhibitory Golgi and the excitatory granule neurons. In addition, our data further show that malfunctioning DDR severely hampers the CNS vasculature, causes myelin damage, and leads to chronic inflammation. The questions mark indicates a suggested process. ML, molecular layer; PCL, Purkinje cell layer; GL, granular layer
| Attachment | Size |
|---|---|
| 950.57 KB |
ABNER C.W. & MCKINNON P.J. (2004): The DNA double-strand break response in the nervous system. DNA Repair (Amst) 3, 1141-1147.
AIELLO L.P. AVERY R.L. ARRIGG P.G. KEYT B.A. JAMPEL H.D. SHAH S.T. PASQUALE L.R. THIEME H. IWAMOTO M.A. PARK, J.E. et al. (1994): Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331, 1480-1487.
AMROMIN G.D. BODER E. & TEPLITZ R. (1979): Ataxia-telangiectasia with a 32 year survival. A clinicopathological report. J Neuropathol Exp Neurol 38, 621-643.
ANDEGEKO Y. MOYAL L. MITTELMAN L. TSARFATY I. SHILOH Y. & ROTMAN G. (2001): Nuclear retention of ATM at sites of DNA double strand breaks. J Biol Chem 276, 38224-38230.
ASSAF Y. GALRON, R. SHAPIRA, I. NITZAN A. BLUMENFELD-KATZIR T. SOLOMON A.S. HOLDENGREBER V. WANG Z.Q., SHILOH Y. & BARZILAI A. (2008): MRI evidence of white matter damage in a mouse model of Nijmegen breakage syndrome. Exp Neurol 209, 181-191.
BAKKENIST C.J. & KASTAN M.B. (2003): DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499-506.
BALDWIN R.C. & O'BRIEN J. (2002): Vascular basis of late-onset depressive disorder. Br J Psychiatry 180, 157-160.
BARBIERI F. SANTORO L. CRISCI C. MASSINI R. RUSSO E. & CAMPANELLA G. (1986): Is the sensory neuropathy in ataxia-telangiectasia distinguishable from that in Friedreich's ataxia? Morphometric and ultrastructural study of the sural nerve in a case of Louis Bar syndrome. Acta Neuropathol 69, 213-219.
BARLOW C. HIROTSUNE S. PAYLOR R. LIYANAGE M., ECKHAUS M. COLLINS F. SHILOH Y. CRAWLEY J.N. RIED T. TAGLE D. et al. (1996): Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159-171.
BARZILAI A. (2011): The neuro-glial-vascular interrelations in genomic instability symptoms. Mech Ageing Dev 132, 395-404.
BARZILAI A. (2013): The interrelations between malfunctioning DNA damage response (DDR) and the functionality of the neuro-glio-vascular unit. DNA Repair (Amst).
BARZILAI A., BITON S. & SHILOH Y. (2008): The role of the DNA damage response in neuronal development, organization and maintenance. DNA Repair (Amst) 7, 1010-1027.
BARZILAI A., SCHUMACHER B. & SHILOH Y. (2016): Genome instability: Linking ageing and brain degeneration. Mech Ageing Dev.
BITON S. BARZILAI A. & SHILOH Y. (2008): The neurological phenotype of ataxia-telangiectasia: solving a persistent puzzle. DNA Repair (Amst) 7, 1028-1038.
BORGHESANI P.R. ALT F.W. BOTTARO, A. DAVIDSON L. AKSOY S. RATHBUN G.A. ROBERTS T.M. SWAT W. SEGAL R.A. & GU Y. (2000): Abnormal development of Purkinje cells and lymphocytes in Atm mutant mice. Proc Natl Acad Sci U S A 97, 3336-3341.
BRADFORD M.M. (1976): A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248-254.
CHRZANOWSKA K.H. GREGOREK H. DEMBOWSKA-BAGINSKA B. KALINA M.A. & DIGWEED M. (2012): Nijmegen breakage syndrome (NBS). Orphanet J Rare Dis 7, 13.
CLAYCOMB K.I. JOHNSON K.M. WINOKUR P.N. SACINO A.V. & CROCKER S.J. (2013): Astrocyte regulation of CNS inflammation and remyelination. Brain Sci 3, 1109-1127.
CORREALE J. & FAREZ M.F. (2015): The Role of Astrocytes in Multiple Sclerosis Progression. Front Neurol 6, 180.
CRAWFORD T.O. (1998). Ataxia telangiectasia. Semin Pediatr Neurol 5, 287-294.
CRAWFORD T.O. MANDIR A.S. LEFTON-GREIF M.A. GOODMAN S.N. GOODMAN B.K. SENGUL H. & LEDERMAN H.M. (2000): Quantitative neurologic assessment of ataxia-telangiectasia. Neurology 54, 1505-1509.
CRAWFORD T.O. SKOLASKY R.L. FERNANDEZ R. ROSQUIST K.J. & LEDERMAN H.M. (2006): Survival probability in ataxia telangiectasia. Arch Dis Child 91, 610-611.
DELUCA G.C. WILLIAMS K. EVANGELOU N. EBERS G.C. & ESIRI M.M. (2006): The contribution of demyelination to axonal loss in multiple sclerosis. Brain 129, 1507-1516.
DETMAR M. BROWN L.F. BERSE B. JACKMAN R.W. ELICKER B.M. DVORAK H.F. & CLAFFEY K.P. (1997): Hypoxia regulates the expression of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) and its receptors in human skin. J Invest Dermatol 108, 263-268.
DIESTEL A. AKTAS O. HACKEL D. HAKE I. MEIER S. RAINE C.S. NITSCH R. ZIPP F. & ULLRICH O. (2003): Activation of microglial poly(ADP-ribose)-polymerase-1 by cholesterol breakdown products during neuroinflammation: a link between demyelination and neuronal damage. J Exp Med 198, 1729-1740.
DIGWEED M. & SPERLING K. (2004): Nijmegen breakage syndrome: clinical manifestation of defective response to DNA double-strand breaks. DNA Repair (Amst) 3, 1207-1217.
EILAM R. PETER Y. GRONER Y. & SEGAL M. (2003): Late degeneration of nigro-striatal neurons in ATM-/- mice. Neuroscience 121, 83-98.
ELSON A. WANG Y. DAUGHERTY C.J. MORTON C.C. ZHOU F. CAMPOS-TORRES J. & LEDER P. (1996): Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc Natl Acad Sci U S A 93, 13084-13089.
FELLIN T. PASCUAL O. GOBBO S. POZZAN T. HAYDON P.G. & CARMIGNOTO G. (2004): Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron 43, 729-743.
FIELDS R.D. ARAQUE A. JOHANSEN-BERG H. LIM, S.S. LYNCH G. NAVE K.A, NEDERGAARD M.PEREZ R. SEJNOWSKI T. & WAKE H. (2014): Glial biology in learning and cognition. Neuroscientist 20, 426-431.
FORSTL H, & HOWARD R. (1991): Recent studies on dementia senilis and brain disorders caused by atheromatous vascular disease: by A. Alzheimer, 1898. Alzheimer Dis Assoc Disord 5, 257-264.
FRENKEL D. PUCKETT L. PETROVIC S. XIA W. CHEN G. VEGA J. DEMBINSKY-VAKNIN A. SHEN J. PLANTE M. BURT D.S. et al. (2008): A nasal proteosome adjuvant activates microglia and prevents amyloid deposition. Ann Neurol 63, 591-601.
GALRON R. GRUBER R. LIFSHITZ V. LU H. KIRSHNER M. ZIV N. WANG Z.Q. SHILOH Y. BARZILAI A. & FRENKEL D. (2011): Astrocyte dysfunction associated with cerebellar attrition in a nijmegen breakage syndrome animal model. J Mol Neurosci 45, 202-211.
GIAUME C. KIRCHHOFF F. MATUTE C. REICHENBACH A. & VERKHRATSKY A. (2007): Glia: the fulcrum of brain diseases. Cell Death Differ 14, 1324-1335.
HALASSA M.M. &HAYDON P.G. (2010): Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol 72, 335-355.
HAN X. CHEN M. WANG F. WINDREM M. WANG S. SHANZ S. XU Q. OBERHEIM N.A. BEKAR L. BETSTADT S. et al. (2013): Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12, 342-353.
HANDE M.P. BALAJEE A.S. TCHIRKOV A. WYNSHAW-BORIS A. & LANSDORP P.M. (2001): Extra-chromosomal telomeric DNA in cells from Atm(-/-) mice and patients with ataxia-telangiectasia. Hum Mol Genet 10, 519-528.
HARHAJ N.S. & ANTONETTI D.A. (2004): Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol 36, 1206-1237.
HARLOW E. & LANE D. (1988): Antibodies: A laboratroy manual (Cold Spring Harbor Laboratroy).
HAYDON P.G. V CARMIGNOTO G. (2006): Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86, 1009-1031.
HERZ J. ZIPP F. & SIFFRIN V. (2010): Neurodegeneration in autoimmune CNS inflammation. Exp Neurol 225, 9-17.
HOFMAN P. VAN BLIJSWIJK B.C. GAILLARD P.J. VRENSEN G.F. & SCHLINGEMANN R.O. (2001): Endothelial cell hypertrophy induced by vascular endothelial growth factor in the retina: new insights into the pathogenesis of capillary nonperfusion. Arch Ophthalmol 119, 861-866.
HUGHES E.G. ELMARIAH S.B. & BALICE-GORDON R.J. (2010): Astrocyte secreted proteins selectively increase hippocampal GABAergic axon length, branching, and synaptogenesis. Mol Cell Neurosci 43, 136-145.
Iliakis G. Wang Y. Guan J. v Wang H. (2003): DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene 22, 5834-5847.
KIRCHHOFF F. DRINGEN R. & GIAUME C. (2001): Pathways of neuron-astrocyte interactions and their possible role in neuroprotection. Eur Arch Psychiatry Clin Neurosci 251, 159-169.
KRUMINIS-KASZKIEL E. WOJTKIEWICZ J. & MAKSYMOWICZ W. (2014): Glial-restricted precursors as potential candidates for ALS cell-replacement therapy. Acta Neurobiol Exp (Wars) 74, 233-241.
KURZ E.U. & LEES-MILLER S.P. (2004): DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amst) 3, 889-900.
LAVIN M.F. (2008): Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 9, 759-769.
LAVIN M.F. & SHILOH Y. (1997): The genetic defect in ataxia-telangiectasia. Annu Rev Immunol 15, 177-202.
LU F. SELAK M. O'CONNOR J. CROUL S. LORENZANA C. BUTUNOI C. & KALMAN B. (2000): Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci 177, 95-103.
MA D.K. MING G.L. & SONG H. (2005): Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol 15, 514-520.
MARCHETTO M.C. MUOTRI A.R. MU Y. SMITH A.M. CEZAR G.G. & GAGE F.H. (2008): Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3, 649-657.
MCKIMMIE C.S. & GRAHAM G.J. (2010): Astrocytes modulate the chemokine network in a pathogen-specific manner. Biochem Biophys Res Commun 394, 1006-1011.
MCKINNON P.J. (2009): DNA repair deficiency and neurological disease. Nat Rev Neurosci 10, 100-112.
MCKINNON P.J. (2012): ATM and the molecular pathogenesis of ataxia telangiectasia. Annu Rev Pathol 7, 303-321.
MESHULA L. GALRON R. KANNER S. DE PITTA M. BONIFAZI P. GOLDIN M. FRENKEL, D. BEN-JACOB E. & BARZILAI A. (2012): The role of the neuro-astro-vascular unit in the etiology of ataxia telangiectasia. Front Pharmacol 3, 157.
MEYN M.S.K. K.K. YOUNG D. AND WANG W. (2003): Nuclear dynamics of ATM following DNA damage.
MOORE C.I. & CAO R. (2008): The hemo-neural hypothesis: on the role of blood flow in information processing. J Neurophysiol 99, 2035-2047.
NAGLER K. MAUCH D.H. & PFRIEGER F.W. (2001): Glia-derived signals induce synapse formation in neurones of the rat central nervous system. J Physiol 533, 665-679.
O'DRISCOLL M. GENNERY A.R., SEIDEL J. CONCANNON P. & JEGGO P.A. (2004): An overview of three new disorders associated with genetic instability: LIG4 syndrome, RS-SCID and ATR-Seckel syndrome. DNA Repair (Amst) 3, 1227-1235.
OKUNO Y. NAKAMURA-ISHIZU A. OTSU K. SUDA T. & KUBOTA Y. (2012): Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nat Med 18, 1208-1216.
PAULA-BARBOSA M.M., RUELA C. TAVARES M.A. PONTES C. SARAIVA A. & CRUZ C. (1983): Cerebellar cortex ultrastructure in ataxia-telangiectasia. Ann Neurol 13, 297-302.
RAKIC P. (1971): Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol 141, 283-312.
RAZ-PRAG D. GALRON R. SEGEV-AMZALEG N. SOLOMON A.S. SHILOH Y. BARZILAI A. & FRENKEL D. (2011): A role for vascular deficiency in retinal pathology in a mouse model of ataxia-telangiectasia. Am J Pathol 179, 1533-1541.
RICCI G. VOLPI L. PASQUALI L. PETROZZI L. & SICILIANO G. (2009): Astrocyte-neuron interactions in neurological disorders. J Biol Phys 35, 317-336.
SATOH J. NAKANISHI M. KOIKE F. MIYAKE S. YAMAMOTO T. KAWA M. KIKUCHI S. NOMURA K. YOKOYAMA K. OTA K. et al. (2005): Microarray analysis identifies an aberrant expression of apoptosis and DNA damage-regulatory genes in multiple sclerosis. Neurobiol Dis 18, 537-550.
SAVITSKY K. BAR-SHIRA A. GILAD S. ROTMAN G. ZIV Y. VANAGAITE L. TAGLE D.A. SMITH S. UZIEL T. SFEZ S, et al. (1995a): A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268, 1749-1753.
SAVITSKY, K. PLATZER M. UZIEL T. GILAD S. SARTIEL A. ROSENTHAL A. ELROY-STEIN O. SHILOH Y. & ROTMAN G. (1997): Ataxia-telangiectasia: structural diversity of untranslated sequences suggests complex post-transcriptional regulation of ATM gene expression. Nucleic Acids Res 25, 1678-1684.
SAVITSKY K. SFEZ S. TAGLE D.A. ZIV Y. SARTIEL A. COLLINS F.S. SHILOH Y. & ROTMAN G. (1995b): The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum Mol Genet 4, 2025-2032.
SHILOH Y. (1995): Ataxia-telangiectasia: closer to unraveling the mystery. Eur J Hum Genet 3, 116-138.
SHILOH Y. (2003): ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3, 155-168.
SHILOH Y. (2014): ATM: Expanding roles as a chief guardian of genome stability. Experimental cell research 329(1), 154-61.
SHWEIKI D. ITIN A. SOFFER D. & KESHET E. (1992): Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843-845.
SOFRONIEW M.V. & VINTERS H.V. (2010): Astrocytes: biology and pathology. Acta Neuropathol 119, 7-35.
STRACKER T.H. & PETRINI J.H. (2011): The MRE11 complex: starting from the ends. Nature reviews Molecular cell biology 12, 90-103.
SU T.T. (2006): Cellular responses to DNA damage: one signal, multiple choices. Annu Rev Genet 40, 187-208.
TAYLOR A.M. GROOM A. & BYRD P.J. (2004): Ataxia-telangiectasia-like disorder (ATLD)-its clinical presentation and molecular basis. DNA Repair (Amst) 3, 1219-1225.
TOLENTINO M.J. MILLER J.W. GRAGOUDAS E.S. JAKOBIEC F.A. FLYNN E. CHATZISTEFANOU K. FERRARA N. & ADAMIS A.P. (1996): Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology 103, 1820-1828.
UENO M. CHIBA Y. MURAKAMI R. MATSUMOTO K. KAWAUCHI M. & FUJIHARA R. (2016): Blood-brain barrier and blood-cerebrospinal fluid barrier in normal and pathological conditions. Brain Tumor Pathol 33, 89-96.
ULLIAN E.M. SAPPERSTEIN S.K. CHRISTOPHERSON K.S. & BARRES B.A. (2001): Control of synapse number by glia. Science 291, 657-661.
UZIEL T. LERENTHAL Y. MOYAL L. ANDEGEKO Y. MITTELMAN L. & SHILOH Y. (2003): Requirement of the MRN complex for ATM activation by DNA damage. Embo J 22, 5612-5621.
VOLTERRA A. & MELDOLESI J. (2005): Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6, 626-640.
XU Y. ASHLEY T. BRAINERD E.E., BRONSON R.T. MEYN M.S. & BALTIMORE D. (1996): Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev 10, 2411-2422.
ZHOU B.B. & ELLEDGE S.J. (2000): The DNA damage response: putting checkpoints in perspective. Nature 408, 433-439.
ZLOKOVIC B.V. (2008): The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178-201.