This review focuses on general optogenetics issues (in particular the choice of the necessary light exposure settings), as well as certain promising areas of research with optogenetics.
Introduction
Optogenetics is a method that combines genetic and optic approaches to control the electrical activity of excitable cells (neurons and muscle fibers) (Deisseroth, 2011).This method is based on implementationof specific light-sensitive proteins (Oesterhelt&Stoeckenius, 1971), which are called opsins. These trans-membrane proteins change their conformation under light with a specific wavelength (390–700 nm) and as a result ionic currents flow across the cell membrane. In turn, positively-charged (cations) or negatively-charged (anions) ionic movements lead to cell depolarization or hyperpolarization. Optogenetics uses different opsins, but preferably Channelrhodopsin-2(Nagelet al., 2003), ArchT andHalorhodopsin (Matsuno-Yagi&Mukohata, 1977). Channelrhodopsin-2 (ChR2, cation channels, responsive to 470 nm wavelength blue light) are applied for excitatory responses. In contrast, inhibitory responses can be evoked by activating Archaerhodopsin and Halorhodopsin (Arch, light-driven proton pump, responsive to 566 nm wavelength yellow light , HR, transmembrane chloride ion-pump, responsive to 580 nm wavelength yellow light).As a result, the activity of neuronal cells can be changed just under the light of a specific wavelength.The idea to use light for selective control of neural activity in different brain cells was formulated by Francis Crick during a lecture at the University of California at San Diego in 1999(Crick, 1979).In 2002, Professor GeroMizenbёkfor the first time postulated the principles of optogenetic method, which allow to control genetically modified nerve cells by the light(Zemelmanet al., 2002). A few years later Karl Deisserothfrom Stanford University described optogenetic technology, caused a great interest in scientific community(Boydenet al., 2005).
After 15 years, optogenetics has found application in various fields, which will be discussed below.At the present time optogenetics is widely applied in neurobiological research. However, there are difficulties in apply it to the clinic. Success of the practical application is determined not only by opsins but also by the selected light mode.
The Importance of Light Mode
The genetic component of optogenetic approach provides the formation of the light-sensitive ion channels. Optical and technical devices deliver light flux with high precision in the specified brain area. In addition,time scale of this manipulation is similar to the characteristics of processes in living organisms and can be adjusted during the experiments. Therefore, it is necessary to analyze which light modes can lead to the positive result in performing various research projects using optogenetics.
The required parametersand modes of the light beam need to be changed according to the purpose of the experiments.In one of the first technical reports (Boyden et al., 2005) wasdemonstrated reliable, millisecond-timescale control of neuronal firing, as well as control of excitatory and inhibitory synaptic transmission in different modes of the light beam (frequency, duration and the parameters of pulse series). The authors described that millisecond pulse of blue light induced single action potentials in ChR2-expressing neurons. Firing activity driven by the activation of this opsin is controlled with high precision at frequencies up to 30 Hz.In another classic article (Zhang et al., 2007) the authors developed the light-driven chloride pump (NpHR) from Natronomonaspharaonis for temporally precise optical inhibition of neural activity. In this case, the intensity and frequency of the light determine the power of firing inhibition.
In the experiments, it is also necessary to consider that the brain tissue of mammals strongly absorb light at 500 microns from the optofiberedge. The intensity of the light beam is approximately 10% of the original intensity (Aravanis, et al., 2007).
A successful exampleof different light mode’s application is the research in motoneurons. The transition from low to higher frequencies of light pulses change character of exposure – from stimulation to inhibition (Liske et al., 2013). The authors demonstrated the control of neuronal excitation and inhibition using light of the same wavelength with varying impulse frequency in the cells expressing one opsin type.
Another research group from Department of Neurobiology and Anatomy (Wake Forest School of Medicine, Winston-Salem, North Carolina, United States) demonstrated that only stimulation with low frequency (5 Hz) leads to the effect in rats with alcohol addiction. This activation resulted in low, but long-term increase in the concentration of dopamine in the nucleus accumbens and led to lossof interest in alcohol (Bass et al., 2013).
Some studies demonstrated that more important than the frequency is the pattern of stimulation. For example, it was shown in the work of (Tye et al., 2013) that mice with depression induced by chronic mild stress, only pulse-phase stimulation has given mental and emotional stability to animals.
Thus, optogenetics provides the opportunity to understand how neurons and neural ensembles are functioning, however, the experimental results will greatly depend on the type of neurons, opsins and stimulation parameters.
Potential Clinic Applications
Optogenetics revolutionized modern experimental neurobiology, making it possible to identify the role of specific neural circuits in the regulation of physiological functions and behavior. Using the necessary opsins and parameters of light effects, as well as the results of functioning of neural circuits, scientists are able to determine the affected circuits for a variety of neurological diseases. Manipulating the activity of neurons in certain areas of the nervous system, responsible for the development of the disease, it is possible to develop a therapeutic strategy, partially or completely eliminating effects of CNS injuries and pathologies.
Optogenetic in Depression
Depression is a serious psychiatric disorder. Despite the long-term study, the underlying mechanisms are still largely unknown (Marsden, 2013). Common antidepressants affect serotonin and noradrenergic neurotransmission and achieve full remission in only 28%. Although they are effective in some patients. As many as 30–40% of patients with major depressive disorder have treatment-resistant depression, which does not respond to currently available antidepressant therapies (Qiao et al., 2015, Monteggia et al., 2014). In our opinion, optogenetic can be applied to treatment of these patients. The changes of neuronal activity in structures of the brain involved in emotion regulation, associated with anxiety, anhedonia, depressed positive emotional reactions correlate with major depression in humans, as well as depression-like behavior in animals (Dygalo, 2015). Several chronic stress models, including chronic restraint stress (CRS), chronic unpredictable mild stress (CUMS), and chronic social defeat stress (CSDS), have been used to recapitulate depression-like behaviors in rodents and study the underlying mechanisms, focusing largely on behavioral tests or aspects of the disorder, such as helplessness or anhedonia (Qiao et al., 2015).
The path to better treatments might more suitably lie in defining the specific brain circuits that mediate symptoms of mental illness. Improved methods for simultaneous recording and manipulating neural activity in the brain’s reward system have led to advances in defining the circuits that mediate several symptoms such as anhedonia. Similarly, circuits involving basolateral amygdala (BLA) neurons or their projections to the central amygdala (CeA) have been found using an optogenetics to mediate features of anxiety, which often accompanies depression, and can modulate conditional fear (Steinberg et al., 2015). This process may ultimately allow to identify targets in neuronal circuits that, when manipulated, can repair the dysfunction (Monteggia et al., 2014).
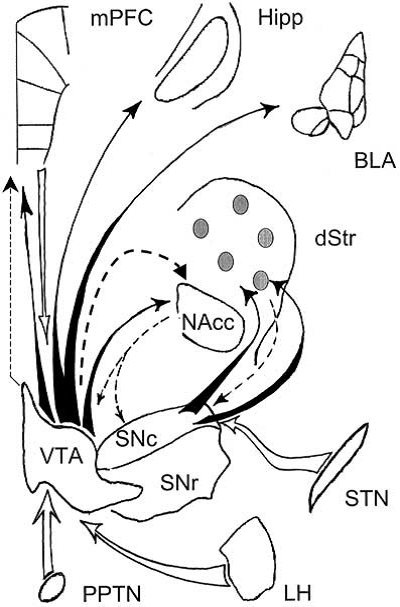
Optogenetic experiments applied for the analysis underlying mechanisms of depression, focused mainly on dopaminergic neurons, especially on neurons of the ventral tegmental area (VTA) (Chaudhury et al., 2013, Tye et al., 2013). Furthermore, changes in the morphology of neurons in the hippocampus, prefrontal cortex, amygdala, and nucleus accumbens established in case patients with depression. All these structures have projections from VTA neurons (Fig. 1) (Korotkovaet al., 2004).
Research on mice using optogenetic approach shows instant behavioral effects in two models of depression in case of stimulation of selectively expressed channelrhodopsin in dopaminergic neurons of ventral tegmental area (Dygalo, 2015). Pulse-phase stimulation recovered psycho-emotional stability, and the inhibition stimulated depressive-like state in the forced swimming and sucrose consumption tests for depression model induced by CUMS (Tye et al., 2013).
Figure 1. A simplified scheme of the connectivity profile of VTA and SNc. Filled arrows indicate dopaminergic projections; dashed arrows - GABAergic; white arrows - glutamatergic pathways. Note that ventral portion of SNc projects to striatal patches (gray circles), while dorsal SNc, to striatal matrix. mPFC - medial prefrontal cortex; hipp - temporal hippocampus; BLA - basolateral complex of the amygdala; Nacc - nucleus accumbens; dStr - dorsal striatum; STN - subthalamic nucleus; PPTN -pedunculopontine tegmental nucleus; LH - lateral hypothalamic area.(Korotkovaet al., 2004)
Taken together understanding chronic stress- and/or depression-induced upstream/downstream signaling pathways and neurocircuits may pave the path for developing efficiency therapeutic strategies for depression. The best strategy for these experiments is to use essential temporal resolution and dignity of optogenetic approach.
Optogenetic in spinal cord injury
Asdescribedaboveoptogeneticsmaybeappliedinneurology, inparticularforthetreatmentof spinal injuries (SCI). Historically, the most common form of stimulus to activate the neuromuscular component was electricity. Namely, a functional electrical stimulation (FES), which has been successfully applied to restore breathing (Glenn et al., 1980), the upper (Cragoet al., 1991) and lower (Ragnarssonet al., 1991) limb function and of the bladder and bowel control(Creasey&Craggs, 2012).Despite the proven efficacy of electrical stimulation, the lack ofactivation control led to the limited integration of FES systems. To solve this problem, can be applied optogenetics, which has an accuracy andhigh time-scale resolution.
Spinal cord injuries disrupted breathing, muscle and lower body function.
Respiration function after SCI. The injuries at the cervical level are the most common after SCI. The advantage of optogenetics in the art that it is relatively less invasive, and has a powerful stimulating action on the nervous system. In a study performed by Alilain et al. (Alilainet al., 2008), they introduced a viral vector containing the ChR2 gene into the gray matter of the spinal cord after SCI in the C2 area. The results showed that occur recovery of muscle and respiratory activity in injured rats after light excitation (Adamantidiset al., 2007).
Muscle function after spinal cord injury. After SCI occurs loss connection between motor and sensory axons of the central and peripheral nervous system occurs after SCI. Electrical stimulation of motor axons in the peripheral nervous system leads to the induction of muscle contraction. It is applied to control the function of the diaphragm (Onders et al., 2009). Because of the peripheral nerves system consists of different sensory and motor axons, FES stimulates nerves indiscriminately, and causes considerable discomfort. Thus, it needs more accurate nerves activation (Bryson et al., 2014). In studies (Towne et al., 2013)ChR2 gene was transduced into the endogenous motor neurons in the mice, the axons of motor neurons were excited by light, as a result, scientists have been able to control muscle function. At the other study (Yohn et al., 2008) the ChR2 gene was transducedinto motoneurons of adult rats. This led to action potential generation in response to a light pulse, and hence led to the recovery of muscle function.
Lower body function after spinal cord injury. This kind of damage leads to the loss of locomotor system function and many other functions. For example, in optogenetic study of SCI, genes of ChR2 and halorhodopsin (NpHR) were introduced into the rat spinal cord before injury(Awad et al., 2013). It was expected that the light activationof ChR2 and NpHR expressing animalsincrease or inhibit activityof the bladder after injury.Animals transfected with ChR2 demonstrated a return of urination. Following transfection with NpHRresulted in a temporary loss of micturition. As a result, the light excitation of ChR2, NpHR enabled to control induction of urinary function after experimentally induced SCI in rats.
Optogenetics application for functional recovery after SCI actively conducted in small animal models. Optogenetics has been applied to study the spinal cord operation, which is responsible for the limb movements (Hägglund et al., 2010). In this study, with optogenetics authors described that glutamatergic neurons in the spinal cord are responsible for rhythm generation and provide the drive in the network needed to obtain flexor-extensor and left-right alternation. Neural photo-stimulation was most efficient in the lower thoracic and upper lumbar spinal cord and worked only when light was directed toward the ventral side of the spinal cord. Additionally, Hägglundand colleagues showed rhythmic activation of selective muscles necessary for locomotion after optical stimulation in transgenic mouse line expressing channelrhodopsin-2 in spinal interneurons (Hägglund et al., 2013).
Animal studies suggest that in the future optogenetics will have great opportunities. However, before optogenetics will be applied in the clinic, it is necessary to solve a number of problems. Firstly, it is necessary to develop safe methods of genetic delivery to target cells. Secondly, it is necessary to adjust the lifetime of introduced genetic material. Third, develop the safe light delivery system in a specific tissue andfinally, develop the stimulation control system for users with the ability to control in real-time (Grahn et al., 2014).
Further development of optogenetic technology could lead to the solution of problems related to the treatment of spinal cord injuries.
Conclusion
Optogenetics occupies a special place in the complex of experimental and therapeutic approaches in neurobiology field, because it gives the possibility of manipulation of normal and pathological neuronal networks that cannot be done by any other techniques.Extension of optogenetic studies, of course, will be interesting and productive direction in case of detection of neurological disease mechanisms and finding the ways to correct them.
| Attachment | Size |
|---|---|
| 332.06 KB |
This work was supported by the Russian Scientific Fund grant 14-25-00024.
ABILEZ O.J., WONG J., PRAKASH R., DEISSEROTH K., ZARINS C.K. & KUHL E. (2011): Multiscale computational models for optogenetic control of cardiac function. Biophys. J., 101, 1326–1334.
ADAMANTIDIS A.R., ZHANG F., ARAVANIS A.M., DEISSEROTH K. & DE LECEA L. (2007): Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature, 450, 420-424.
ALILAIN W.J., LI X., HORN K.P., DHINGRA R., DICK T.E., HERLITZE S. & SILVER J. (2008): Light-induced rescue of breathing after spinal cord injury. J.Neurosci, 28, 11862-70.
ARAVANIS A.M., WANG L.P., ZHANG F., MELTZER L.A., MOGRI M.Z., SCHNEIDER M.B. & DEISSEROTH K. (2007): An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng., 4, 143–156.
ARTAMONOV D.N., KORZHOVA V.V., WU J., RYBALCHENKO P.D., IM C., KRASNOBOROVA V.A., VLASOVA O.L. & BEZPROZVANNY I.B. (2013): Characterization of synaptic dysfunction in an in vitro corticostriatal model system of Huntington's disease. Biological Membranes, 30, 276-288.
ASANO T., ISHIZUKA T. & YAWO H.(2013): Muscular optogenetics: controlling muscle functions with light. Nova Science Publishers, 127-136.
AWAD B.I., GUTIERREZ D.V., ALILAIN W. & STEINMETZ M.P. (2013): Optogenetic photostimulation to control bladder function after experimental spinal cord injury. Spine J., 13, S12.
BASS C.E., GRINEVICH V.P., GIOIA D., DAY-BROWN J.D., BONIN K. D., STUBER GARRET D., WEINER J.L. & BUDYGIN E. A. (2013): Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front. Behav. Neurosci.,7, 173.
BOYDEN E.S., ZHANG F., BAMBERG E., NAGEL G. & DEISSEROTH K. (2005): Millisecond-timescale, genetically targeted optical control of neural activity. Nat.Neurosci., 8, 1263–1268.
BRYSON J.B., MACHADO C.B., CROSSLEY M., STEVENSON D., BROS-FACER V., BURRONE J., GREENSMITH L. & LIEBERAM I. (2014): Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science; 344, 94-97.
CASTANEDA P., MUNOZ M., GARCIA-ROJO G., ULLOA J. L., BRAVO J. A., MARQUEZ R., GARCIA-PEREZ M. A., ARANCIBIA D., ARANEDA K., ROJAS P. S., MONDACA-RUFF D., DIAZ-VELIZ G., MORA S., ALIAGA E. & FIEDLER J. L. (2015): Association of N-Cadherin Levels and Downstream Effectors of Rho GTPases With Dendritic Spine Loss Induced by Chronic Stress in Rat Hippocampal Neurons. Journal of Neuroscience Research, 10, 1476-1491.
CHAUDHURYD., WALSHJ. J., FRIEDMANA. K., JUAREZB., KUS. M., WOOK KOOJ.A., FERGUSOND., TSAIH.-CH., POMERANZL., CHRISTOFFELD. J., NECTOWA. R., EKSTRANDM., DOMINGOSA., MAZIE-ROBISONM., MOUZONE., LOBOM. K., NEVER. L., FRIEDMANJ. M., RUSSOS. J., DEISSEROTHK., NESTLER E. J. & HAN M.-H. (2013): Rapid regulation of depression-related behaviors by control of midbrain dopamine neurons. Nature,493, 532–536.
CRAGO P.E., NAKAI R.J. & CHIZECK H.J. (1991): Feedback regulation of hand grasp opening and contact force during stimulation of paralyzed muscle. IEEE Trans Biomed Eng., 38, 17–28.
CREASEY G.H., CRAGGS M.D. (2012): Functional electrical stimulation for bladder, bowel, and sexual function. Handb.Clin. Neurol., 109, 247–257.
Crick F.H. (1979): Thinking about the brain. Sci. Am.,241, 219–232.
DEISSEROTH K. (2010): Controlling the brain with light. Sci. Am., 303, 48-55.
DEISSEROTH K. (2011): Optogenetics. Nature Methods, 8, 26–29.
DYGALO N.N. (2015): Optogenetics in Investigations of Brain Mechanisms of Behavior. Uspekhifiziologicheskikhnauk, 2, 17-23.
ENTCHEVA E. (2013): Cardiac optogenetics. Am. J. Physiol. Heart Circ. Physiol., 304, 1179-1191.
GLENN W.W., HOGAN J.F. & PHELPS M.L. (1980): Ventilatory support of the quadriplegic patient with respiratory paralysis by diaphragm pacing. Surg.Clin. North Am.,60, 1055–1078.
GRAHN P.J., MALLORY G.W., BERRY B.M., HACHMANN J.T., LOBEL D.A. & LUJAN J.L. (2014): Restoration of motor function following spinal cord injury via optimal control of intraspinalmicrostimulation: toward a next generation closed-loop neural prosthesis. Front Neurosci., 1–37.
Gu L., Uhelski M.L., Anand S., Romero-Ortega M., Kim Y.T., Fuchs P.N. &Mohanty S.K.(2015): Pain Inhibition by Optogenetic Activation of Specific Anterior Cingulate Cortical Neurons. Plos one, 10, e0117746.
HÄGGLUND M., BORGIUS L., DOUGHERTY K.J. & KIEHN O. (2010): Activation of groups of excitatory neurons in the mammalian spinal cord or hindbrain evokes locomotion. Nature Neuroscience, 13, 246–252.
HÄGGLUND M., DOUGHERTY K.J., BORGIUS L., ITOHARA S., IWASATO T. & KIEHN O. (2013): Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc Natl AcadSci USA, 110, 11589–11594.
JESSUP M., GREENBERG B., MANCINI D., CAPPOLA T., PAULY D.F., JASKI B., YAROSHINSKY A., ZSEBO K.M., DITTRICH H. & HAJJAR R.J. (2011): Calcium Upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation, 124, 304–313.
JIA Z., VALIUNAS V., LU Z., BIEN H., LIU H., WANG H.Z., ROSATI B., BRINK P.R., COHEN I.S. & ENTCHEVA E. (2011): Stimulating cardiac muscle by light: cardiac optogenetics by cell delivery. Circ. Arrhythm. Electrophysiol.,4, 753–760.
KOROTKOVA T. M., PONOMARENKO A. A., BROWN R. E. & HAAS H. L. (2004): Functional Diversity of Ventral Midbrain Dopamine and GABAergic Neurons. Molecular Neurobiology,3, 243-259.
LISKE H., QIAN X., ANIKEEVA P., DEISSEROTH K. & DELP S. (2013): Optical control of neuronal excitation and inhibition using a singlopsine protein, ChR2. Sientific Reports, 3, 3110.
MARSDEN W. N. (2013): Synaptic plasticity in depression: molecular, cellular and functional correlates. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 43, 168–184.
Matsuno-Yagi A. &Mukohata, Y. (1977): Two possible roles of bacteriorhodopsin; a comparative study of strains of Halobacteriumhalobium differing in pigmentation. Biochem. Biophys. Res. Commun.,78, 237–243.
MONTEGGIA L.M., MALENKA R. C.& DEISSEROTH K. (2014): Depression: The best way forward. Nature,515, 200-201.
NAGEL G., SZELLAS T., HUHN W., KATERIYA S., ADEISHVILI N., BERTHOLD P., OLLIG D., HEGEMANN, P. & BAMBERG E. (2003): Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci., 100, 13940–13945.
OESTERHELT D.& STOECKENIUS W. (1971): Rhodopsin-like protein from the purple membrane of Halobacteriumhalobium. Nat. New Biol.,233, 149–152.
ONDERS R.P., ELMO M., KHANSARINIA S., BOWMAN B., YEE J., ROAD J., BASS B., DUNKIN B., INGVARSSON P.E. & ODDSDÓTTIR M. (2009): Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg.Endosc., 23, 1433-40.
PARKER K.L., KIM Y., ALBERICO S.L., EMMONS E.B. & NARAYANAN N.S. (2016): Optogenetic approaches to evaluate striatal function in animal models of Parkinson disease. Dialogues Clin.Neurosci.,18, 99–107.
QIAO H., LI M.-X., XU C., CHEN H.-B., AN S.-C. & MA X.-M. (2015): Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plasticity, 2016-2026.
RAGNARSSON K.T., POLLACK S.F. & TWIST D. (1991): Lower Limb Endurance Exercise after Spinal Cord Injury: Implications for Health and Functional Ambulation. Neurorehabil Neural Repair,5, 37–48.
SONG J., AMPATZIS K., BJÖRNFORS E.R. & MANIRA A. (2016): Motor neurons control locomotor circuit function retrogradely via gap junctions. Nature, 529, 399–402.
STEINBERG E.E., CHRISTOFFEL D.J., DEISSEROTH K. & MALENKA R.C.(2015): Illuminating circuitry relevant to psychiatric disorders with optogenetics. Current Opinion in Neurobiology, 30, 9-16.
TOWNE C., MONTGOMERY K.L., IYER S.M., DEISSEROTH K. & DELP S.L. (2013): Optogenetic control of targeted peripheral axons in freely moving animals. PLoS One, 8, e72691.
TYE K.M., MIRZABEKOV J.J., WARDEN M.R., FERENCZI E.A., TSAI H.C., FINKELSTEIN J., KIM S.Y., ADHIKARI A., THOMPSON K.R., ANDALMAN A.S., GUNAYDIN L.A., WITTEN I.B. & DEISSEROTH K. (2013): Dopamine neurons modulate neural encoding and expression of depression-related behavior. Nature, 493, 537–541.
TYE K.M., MIRZABEKOV J.J., WARDEN M.R., FERENCZI E.A., TSAI H.-CH., FINKELSTEIN J., KIM S.-Y., ADHIKARI A., THOMPSON K.R., ANDALMAN A.S., GUNAYDIN L.A., WITTEN I.B. & DEISSEROTH K. (2013): Dopamine neurons modulate neural encoding and expression of depression-related behavior. Nature,493, 537–541.
YOHN D.C., MILES G.B., RAFUSEV.F. & BROWNSTONE R.M. (2008): Transplanted mouse embryonic stem-cell-derived motoneurons form functional motor units and reduce muscle atrophy. J.Neurosci., 28, 12409-12418.
ZEMELMAN B.V., LEE G.A., NG M. & MIESENBOCK G. (2002): Selective photostimulation of genetically chARGed neurons. Neuron, 33, 15–22.
ZHANG F., WANG L.P., BRAUNER M., LIEWALD J.F., KAY K., WATZKE N., WOOD P.G., BAMBERG E., NAGEL G., GOTTSCHALK A. & DEISSEROTH K. (2007): Multimodal fast optical interrogation of neural circuitry. Nature, 446, 633–639.