Given the recent findings on the importance of CD38 signaling in the pathogenesis of colon cancer. We hypothesized that single nucleotide polymorphisms (SNP) in the CD38 gene may be related to colon cancer risk. CD38 has a genetic polymorphism, characterized by a C>G variation in the regulatory region of intron 1. The working hypothesis is that the presence of different alleles in colon cancer patients accounts for some of the clinical heterogeneity. CD38 is considered a marker of prognosis and as an indicator the activation and proliferation of cells. We hypothesized that single nucleotide polymorphisms (SNP) in the CD38 gene may be related to colon cancer risk. We evaluated one potentially functional CD38 SNP, intronic rs6449182 in two cases patients and controls. Genotyping was done using PCR-based assays in a total of 93 patients with colon cancer and 100 controls. We found that frequencies of variant allele (rs6449182 G) were significantly higher in colon cancer. Logistic regression analysis revealed an association between colon cancer and genotypes: rs6449182 CC [odds ratio (OR), 0.57; 95% confidence interval (95% CI), 0.32 – 1.01], rs6449182 CG (OR, 1.47; 95% CI, 0.83 – 2.60), and rs6449182 GG (OR, 2.26; 95% CI, 0.66 – 7.77). We observed that rs6449182 G carriers had more advanced clinical stage (P = 0.04). In conclusion, our data show that CD38 SNP may affect CD38 expression and contribute to the increased risk of colon cancer carcinogenesis.
Introduction
Colorectal cancer (CRC) remains one of the most widespread malignancies in the world. According to the last global oncoepidemiological analysis1, 2, 3, although, it has been estimated that worldwide there are 1.2 million new cases a year. Globally, CRC is the fourth most common cause of cancer related deaths in men and third in women, killing an estimated 320,600 men and 288,100 women annually3. The conventional prognostic factors for patient survival are histologic tumor grade (differentiation) and tumor stage (TNM, tumors/nodes/metastases, stages I–IV) 4, 5. CRC is less common in people under the age of 50 years, with the median age at diagnosis being 70 6. Mutations in several different genes seem to be needed to cause CRC. Human CD38 is a 45 kDa single-chain transmembrane glycoprotein with a short amino-terminal cytoplasmic tail, a single membrane-spanning region, and a long extracellular carboxy-terminal domain, the carboxyl-terminal of the molecule harbors the catalytic site (CD38 is defined as an ecto-enzyme) and the binding site for CD31, the non-substrate CD38 ligand7. The molecule may also exist in a soluble form present in biologic fluids in normal, paraphysiologic, and pathologic conditions8. The gene that encodes human CD38 has been mapped to chromosome 4 by means of somatic cell genetics9. More recently, the sub-chromosomal localization of the human CD38 gene (4pl5) has been achieved in the course of studies aimed at the genetic analysis of the molecule10. Human CD38 gene encodes a transmembrane glycoprotein, which is considered a marker of lymphocyte activation and possesses enzymatic activity against ADP-ribose11. Recent studies demonstrated that B-CLL patients can be subdivided into two groups depending on the presence or absence of CD38 on malignant cells12. In studies of the association between CD38 and colon cancer, we analyzed 1 single nucleotide polymorphisms (SNP). SNP is a single nucleotide variation in genomic DNA rare allele frequency of at least 1% 13. SNP rs6449182 located at the 5'-end of the first intron and can affect the expression of CD38 gene. rs6449182, that leads to the presence or absence of a PvuII restriction site. The SNP is located within a putative E-box, a region of binding of the E proteins with a consequent regulation of gene transcription. In the B cell compartment a relevant role is played by E2A that controls the expression of several B lineage genes. E2A was demonstrated to bind to the E-box of the CD38 gene, regulating its expression, and the binding of the protein is influenced by the CD38 genotype, with the G allele resulting in a stronger binding of E2A 14. It is shown that the SNP rs6449182 GG genotype increased the risk of developing cell chronic leukemia15. However, we analyzed CD38 gene SNP for estimate the genetic contributions to complex diseases, such as colon cancer.
Materials and Methods
Patient material and normal controls. The study included 100 samples of peripheral blood of healthy donors, 93 peripheral blood samples of patients with colon cancer from Nizhny Novgorod. Among the patients with CRC, there were 52 (56%) males and 41 (44%) females with a median age of 62 years (range, 42-84). The stage distribution for colon cancer is similar for both sexes.
CD38 genotyping Genomic DNA used for CD38 genotyping was isolated from peripheral blood samples in 93 patients and 100 controls. DNA was isolated by phenol-chloroform extraction. SNP rs6449182 gene CD38 was detected by allele-specific PCR. For each sample, two separate PCR reactions were carried out by using the primers F 5′- CCGCCGGGTGGTGCTGAGTA-3′ (forward), RC 5′-ACGCTCGGTGCCAAGGCCAG-3′ (reverse) and RG 5′-ACGCTCGGTGCCAAGGCCAC-3′ (reverse). Amplification conditions were: denaturation at 94 °C for 3 min, followed by 45 cycles of 94 °C for 30sec, 72 °C for 29 sec, and 72 °C for 1 sec, followed by an extension step of 72 °C for 5 min on an Authorized Thermal Cycler. In all patients, genomic DNA was isolated from peripheral blood at the time of diagnosis. SNPs included in this investigation were chosen based on the literature review and their potential functionality. At the time of the study designing the literature search did not reveal any links between CD38 SNPs and colon cancer. The SNP rs6449182 is located at the 5′-end of intron 1 in the proximity of the CpG island and retinoid acid–responsive element, and thus in the region potentially involved in the regulation of CD38 gene expression16. The rs6449182 genotypes were detected following electrophoresis in a 1.5% and identified as rs6449182 CC or GG homozygous or CG heterozygous in case of the presence of a product in respective tube or as rs6449182 CG heterozygous if products were shown in both tubes.
Statistical analysis Accordance with the Hardy-Weinberg equilibrium within each case and control group was analyzed by χ2 test. For all calculations, P < 0.05 was considered significant. The practical application of this research is related development of methods for predicting the course of disease. Undoubted progress in this direction has been made in the field of onco-hematology 17, 18.
Results and Discussion
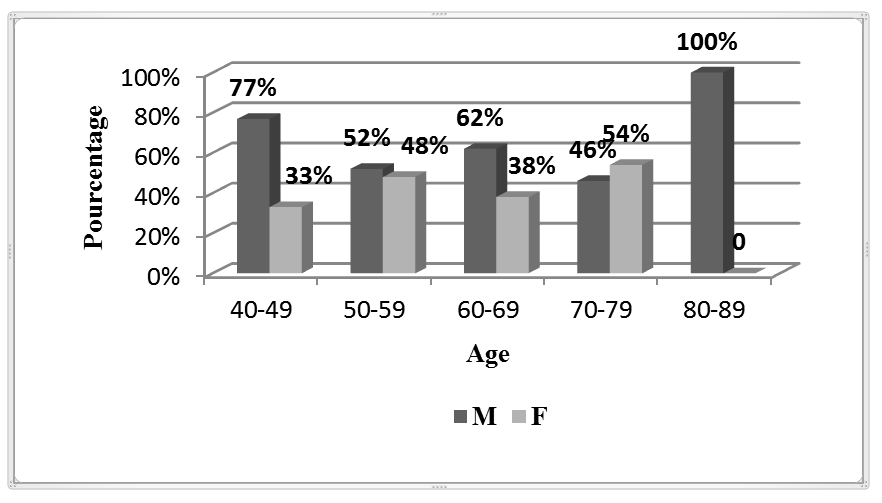
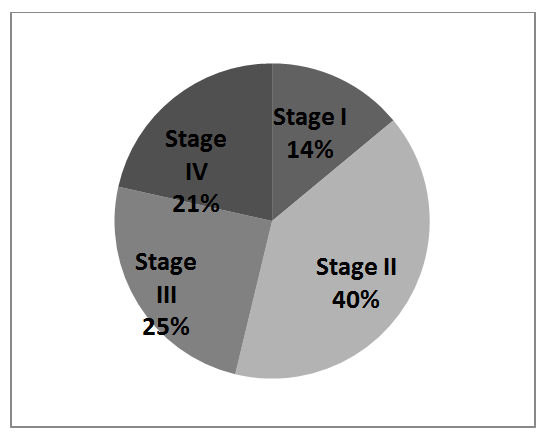
Our studies shows that men tend to develop colon cancer at an earlier age than women with a percentage of 52% men and 41% women (figure. 1), As with most cancers, the risk of colorectal cancer increases with age. Before 40 years, colorectal cancers are very rare. The risk starts to increase from 50 years to 80 years. According to Canadian Cancer Society in 201019, the incidence of CRC increases with age and is higher in men than in women. Compared to the US, and Canada there is a convergent percentage of CRCs diagnosed, data using the same classification as used in the US and Canada (23.2%, 19.7% vs. 20%) 20 (figure. 2).
Healthy donors living in the Nizhny Novgorod region had the following distribution of allelic variants of SNPs rs6449182: CC 0.580, 0.380 CG, and GG 0.040. Investigation frequency of allelic variants of SNP rs6449182 in patients with colon cancer, showed the following distribution: 0.441 CC, CG 0.473, GG 0.86. We are conducting a study of samples of healthy donors and cancer patients on the deviation from Hardy-Weinberg equilibrium. Both samples showed compliance with Hardy-Weinberg equilibrium. No differences in the incidence of genotypic variants of SNPs rs6449182 cancer patients and healthy individuals have been identified. However, it was found that the G allele in patients with colon cancer occurs significantly more likely than healthy donors (χ2 = 4.44, p = 0.04).In our study, regarding rs6449182 SNP, we observed an association between colon cancer and heterozygous CG genotype with an OR of 1.47 (95% CI, 0.83 – 2.60). Interestingly, the colon cancer risk was further elevated with rs6449182 GG homozygous genotype (OR, 2.26; 95% CI, 0.66 – 7.77), suggesting an allele-dose effect. Given the recent findings on the importance of CD38, it is conceivable that polymorphisms of the molecules involved in the CD38 signaling pathway might serve as determinants of colon cancer predisposition. In this investigation, we found that the associations between colon cancer risk and CD38 SNPrs6449182. According to our results the gene frequencies in the healthy population are 0, 77 and 0, 23 for the C and G allele respectively (CC 58%, GC 38% and GG 4%), these results are comparable with data derived from larger series from Spain (CC 53%, GC 40% and GG 7%) with frequencies of C 0,73 and G 0,23 21,Ireland (CC 64%, GC 30% and GG 6%) with frequencies of C 0,79 and G 0,21 22 or Italy(CC 62%, GC 34% and GG 4%) with frequencies of C 0,79 and G 0,21 23. The results obtained show that the G allele of the gene CD38 rs6449182 SNP is likely linked to colon cancer. Our data are consistent with the literature, which shows the role of GG genotype in the risk of chronic leukemia. However, the results should be interpreted with caution. The analysis of this polymorphism in a large cohort of Chronic lymphocytic leukemia (CLL) patients indicate that the G allele is significantly associated with molecular markers of unfavorable prognosis and represents a significant risk factor for RS transformation because the gene frequencies in the healthy population are 0, 78 and 0, 22 for the C and G allele respectively (CC 61%, GC 33% and GG 6%) 23. The correlation between this polymorphism and genetic susceptibility has been studied also for other diseases, including Systemic Lupus Erythematosus (SLE), where the CC genotype causes susceptibility and the CG genotype confers protection for discoid rash development24. One of these tools is CD38, a reliable and independent negative prognostic marker25, which is also involved in the delivery of growth and survival signals to the neoplastic cells26. In this context, the analysis of CD38 polymorphism in CRC patients may offer insights into the regulation of the molecule’s expression, and it may provide clinicians with a novel diagnostic/prognostic marker.
Figure 1. Patient's distribution by age and sex
Conclusions
The biological mechanisms underlying these associations are unknown. Interestingly, we found that CD38 gene and protein expression are elevated in colon cancer cells from carriers of rs6449182 G. The marked heterogeneity observed in that study could now be attributed to the quantitative presence or absence of the G allele. A reading of these results from a clinical standpoint these results suggest that the G allele may represent an independent risk factor for CRC development with potential relevance as a prognostic tool. This is in line with growing evidence of the involvement of CD38 signaling in colon cancer pathogenesis. It was shown that signals through CD38 receptors induce proliferation and increase survival of colon cancer cells27.The rs6449182 polymorphisms is non-coding, although its localization in the regulatory region at the 5′-end of intron 1 in proximity to the CpG island and retinoid acid–responsive element may potentially affect gene expression28. Furthermore, it is not unlikely that association between colon cancer and CD38 SNP may be modified by well-known functional SNP27. The major strengths of our investigation are the design, which has enabled the results to be confirmed in a validation study, and functional findings that are in line with the observed disease associations. However, some limitations of the study should also be mentioned. It should also be underlined that we applied the candidate gene approach, and focused on SNP with functional effects as suggested by previous reports. Therefore, we cannot rule out the possibility that CD38 SNP other than those included in our study may be related to colon cancer risk. In conclusion, in this study, we found some evidence that CD38 SNP rs6449182 affects CD38 expression in colon cancer cells and contributes to colon cancer predisposition.
Figure 2. Stage distribution of Colon Cancer Cases from Nizhny Novgorod
| Attachment | Size |
|---|---|
| 310.92 KB |
Ferlay J., Shin H. R., Bray F., et al. Cancer incidence and mortality worldwide: IARC CancerBase No. 10 [Internet]; 2010. Lyon (France): IARC; Available from: http://globocan.iarc.fr .
Jemal A., Center M. M., DeSantis C., Ward E. M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers; 2010.19: p 1893-1907.
Jemal A., Bray F., Center M. M., et al. Global cancer statistics. CA Cancer J Clin; 2011. 61: p 69-90.
Puppa G, Sonzogni A, Colombari R, Pelosi G. TNM staging system of colorectal carcinoma: A critical appraisal of challenging issues. Arch. Pathol. Lab. Med. 2010;134: p 837–852.
Zinkin LD. A critical review of the classifications and staging of colorectal cancer. Dis. Colon. Rectum.1983;26: p 37–43.
Robin P. Boushey M.D., Ph.D. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors Colorectal Cancer Clin Colon Rectal Surg. 2009 Nov; 22(4): p 191–197. http://cebp.aacrjournals.org/content/18/3/945.full.pdf+html
Deaglio S., Morra M., Mallone R., Ausiello C. M., Prager E., Garbarino G., Dianzani U., Stockinger H., Malavasi F. J Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an Ig superfamily member. Immunol; 1998.160: p 395-402.
Funaro A., Horenstein A. L., Calosso L., et al. Identification and characterization of an active soluble form of human CD38 in normal and pathological fluids. Int Immunol; 1996. 8: p 1643-1650.
Katz F., Povey S., Parkar M., Schneider C., Sutherland R., Stanley K., Solomon E., and Creaves M. Chromosome assignment of monoclonal antibody-defined determinant on human leukemic cells. Eur J. Immunol; 1983. 13: p 1008-1013
Nakagawara K., Mon M., Takasawa S., Nata K., Takamura I., Berlova A., Tohgo A., Karasawa I., Yonekura H., Takeuchi I., and Okamoto H. Assignment of CD38, the gene encoding human leukocyte antigen CD38 (ADP-ribosyl cyclase/cyclic ADP rihose hydrolase), to chromosome 4p15. Cytogene:. Cell. Genet; 1995. 69: p 38-39.
Deaglio. S., Mallone. R, Baj G., Donati D., Giraudo G., Corno F., Bruzzone S., Geuna M., Ausiello C., and Malavasi F. Human CD38 and its ligand CD31 define a unique lamina propria T lymphocyte signaling pathway. FASEB J; 2001.15: p 580-582.
Zupo S., Isnardi L., Megna M., Malavasi F., Dono M., Cosulich E., and Ferrarini M. CD38 expression distinguishes two groups of B cells chronic lymphocytic leukemia with different response to anti-lgM antibodies and propensity to apoptosis. Blood In press; 1996. 88: p 1365-1374.
Mohd F., Mohammad A. Single nucleotide polymorphism in genome-wide association of human population: A tool for broad spectrum service; 2013. 14: p 123–134.
Saborit V. I., Vaisitti T., Rossi D., D'Arena G., Gaidano G., Malavasi F., Deaglio S. E2A is a transcriptional regulator of CD38 expression in chronic lymphocytic leukemia. Leukemia; 2011. 25: p 479-88.
Jamroziak K., Szemraj Z., Izydorczyk O. G., Szemraj J., Bieniasz M., Cebula B., Giannopoulos K., Balcerczak E., Kupnicka D J., Kowal M., Kostyra A., Calbecka M., Wawrzyniak E., Mirowski M., Kordek R and Robak T. CD38 Gene Polymorphisms Contribute to Genetic Susceptibility to B-Cell Chronic Lymphocytic Leukemia: Evidence from Two Case-Control Studies in Polish Caucasians. Cancer Epidemiol Biomarkers; 2009. 18: p 945.
Ferrero E., Saccucci F., Malavasi F. The human CD38 gene: polymorphism, CpG island, and linkage to the CD157 (BST-1) gene. Immunogenetics; 1999. 49: p 597–604.
Novikov V.V. Soluble forms of hemopoietic cells differentiation antigens (Растворимые формы дифференцировочных антигенов гемопоэтических клеток) // Гематология и трансфузиология; 1996. № 6: p 40–43.
Novikov V.V. Soluble differentiation antigens (Растворимые дифференцировочные антигены) // Иммунотерапия рака: Матер. Европ. школы онкологов. М; 1999: p 1–8.
The Canadian Partnership Against Cancer This document was produced in November 2010 and can be found at: www.cancerview.ca
Canadian Cancer Society. Canadian Cancer Statistics 2010. Toronto 2010.
Gonzalez E. M. F., Aguilar F., Torres B., Sanchez R. J, Nunez R. A. CD38 polymorphisms in Spanish patients with systemic lupus erythematosus. Hum Immunol; 2004. 65: p 660- 664.
Drummond F. J., Mackrill J. J., O’Sullivan K., Daly M., Shanahan F., Molloy M. G. CD38 is associated with premenopausal and postmenopausal bone mineral density and postmenopausal bone loss. J Bone Miner Metab; 2006. 24: p 28-35.
Aydin S., Rossi D., Bergui L., D'Arena G., Ferrero E., Bonello L., Omede P., Novero D., Morabito F., Carbone A., Gaidano G., Malavasi F., Deaglio S. CD38 gene polymorphism and chronic lymphocytic leukemia: a role in transformation to Richter syndrome? Blood; 2008. 111: p 5646-5653.
Gonzalez E. M. F., et al. Idem; 2004. 65: p 660- 664.
Matrai Z. CD38 as a prognostic marker in CLL. Hematology. 2005;10: p 39-46.
Deaglio S, Vaisitti T, Aydin S, Ferrero E, Malavasi F. In-tandem insight from basic science combined with clinical research: CD38 as both marker and key component of the pathogenetic network underlying chronic lymphocytic leukemia. Blood. 2006;108: p1135-1144.
Deaglio S., Capobianco A., Bergui L., et al. CD38 is a signaling molecule in B-cell chronic lymphocytic leukemia cells. Blood; 2003. 102: p 2146–2155.
Ferrero E., et al. Idem;1999. 49: p 597–604.
Sasaoka T., Kimura A., Hohta S. A., Fukuda N., Kurosawa T., Izumi T. Polymorphisms in the platelet-endothelial cell adhesion molecule-1 (PECAM-1) gene, Asn563Ser and Gly670Arg, associated with myocardial infarction in the Japanese. Ann N Y Acad Sci; 2001. 947: p 259–270.