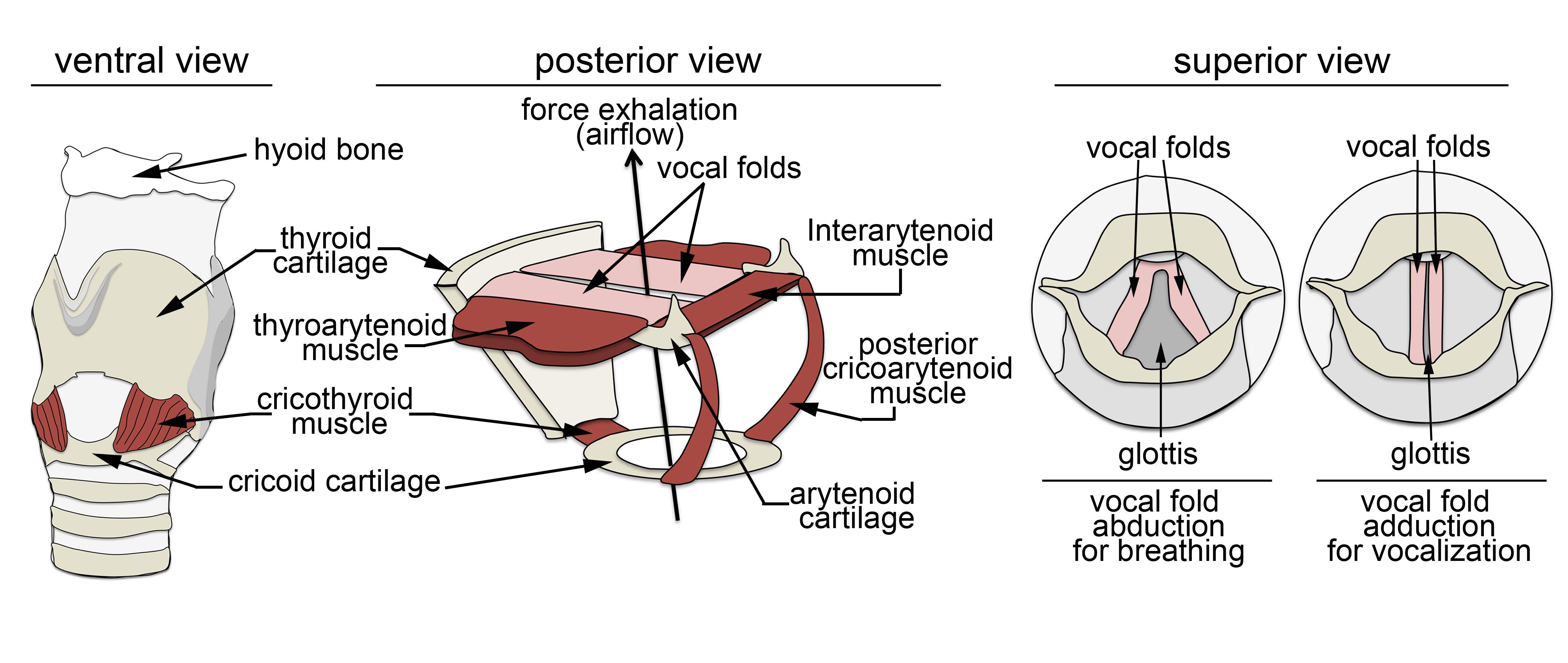
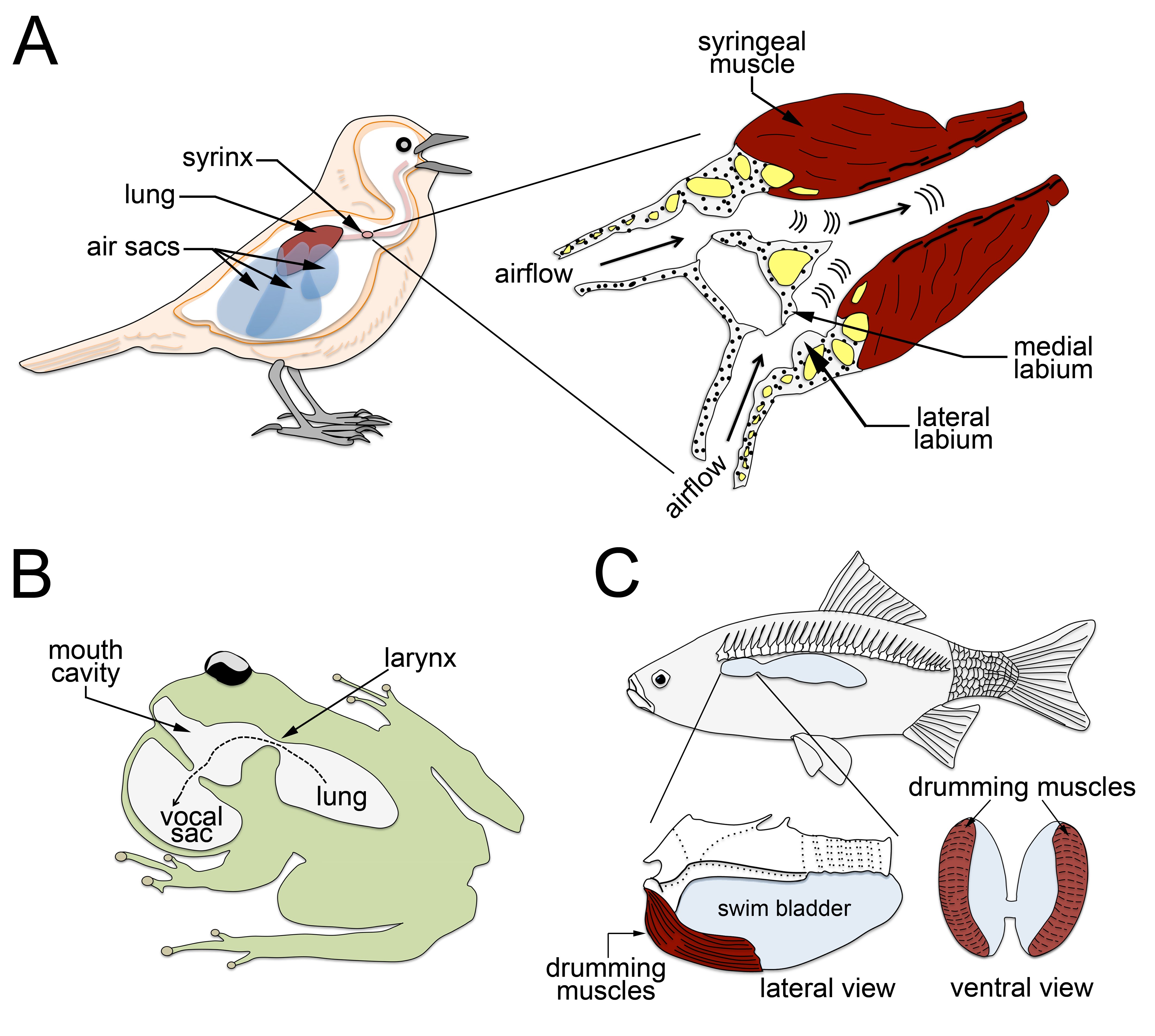
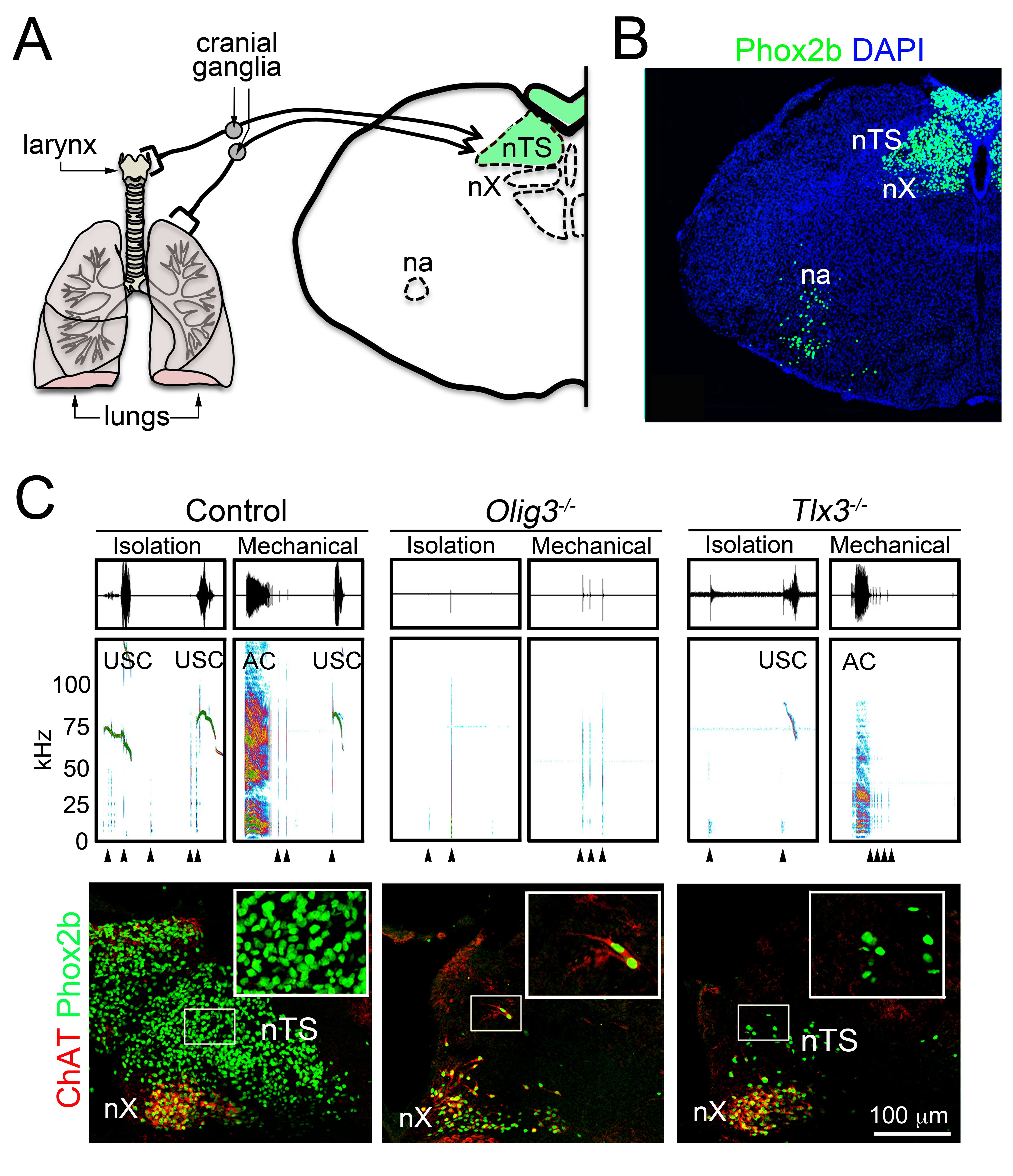
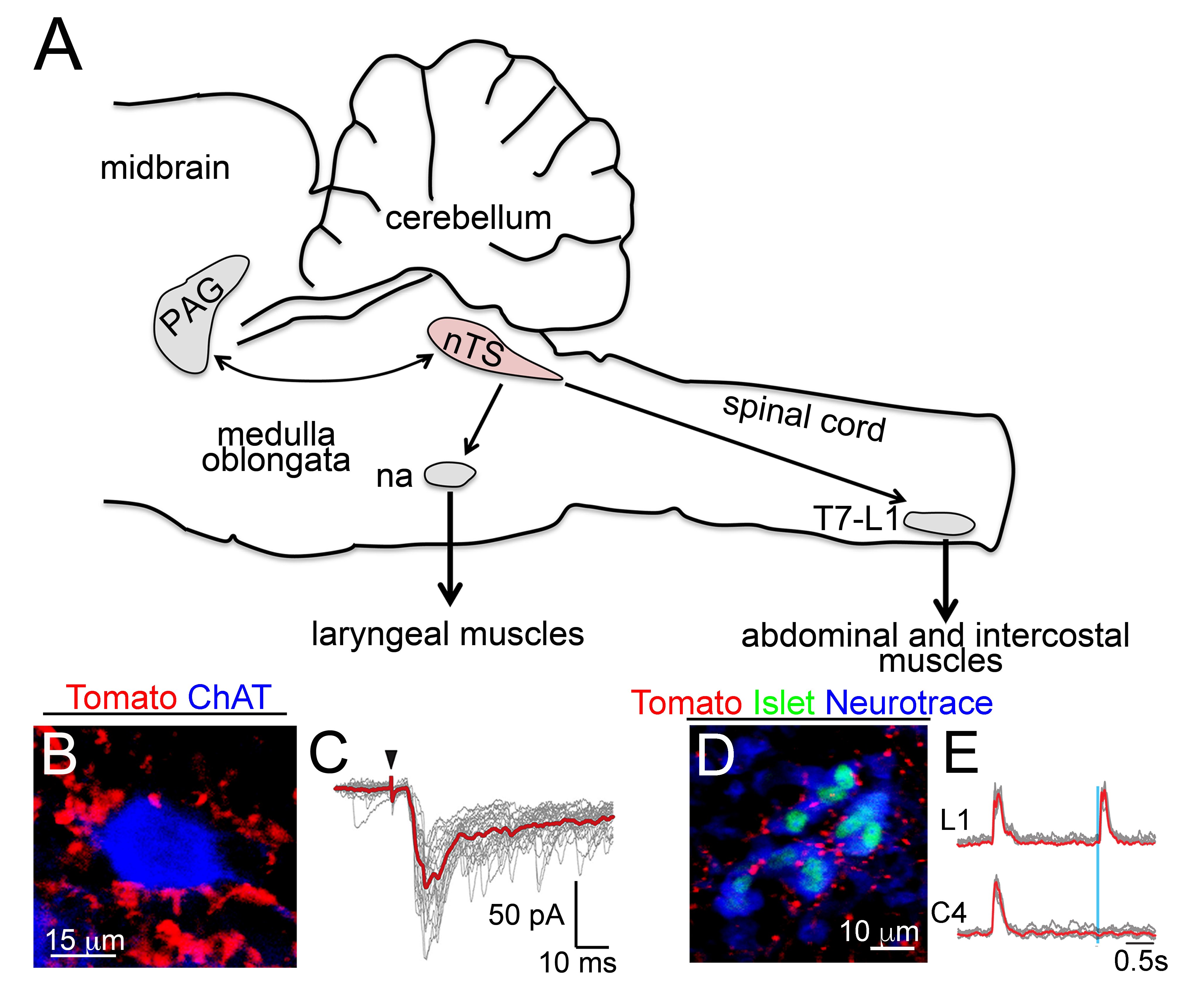
Vocalization is a highly conserved innate behavior in vertebrates. It is mainly used in social encounters to communicate a variety of information for inter- and intra- specific interactions. In this review, we focus on the anatomical, biomechanics and neuronal circuits underlying vocalization across vertebrate species. In addition, we discuss our recent findings that assign to the nucleus of the solitary tract a critical role in innate vocalization. This brain center receives viscerosensory information, i.e. information from internal organs that includes the lungs and the larynx. Furthermore, subpopulations of neurons in the nucleus of the solitary tract directly connect to and entrain the activity of expiratory and laryngeal motor neurons. In mammals and amphibians, these motor neurons control essential biomechanical parameters used for vocalization, and similar motor neuron pools regulate vocal utterances in birds. Thus vocalization relies on a conserved neuronal circuit residing in the brainstem and spinal cord.
| Attachment | Size |
|---|---|
| 6.86 MB |
Abdala, A.P., Rybak, I.A., Smith, J.C., Paton, J.F., 2009. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol 587, 3539-3559.
Adametz, J., O'Leary, J.L., 1959. Experimental mutism resulting from periaqueductal lesions in cats. Neurology 9, 636-642.
Alheid, G.F., Jiao, W., McCrimmon, D.R., 2011. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neuroscience 190, 207-227.
Allen, E., Murcek, B.W., 2018. Anatomy, Neck, Larynx, Nerves, Recurrent Laryngeal, StatPearls, Treasure Island (FL).
Apfelbach, R., 1972. Electrically elicited vocalizations in the gibbon Hylobates lar (Hylobatidae), and their behavioral significance. Z Tierpsychol 30, 420-430.
Bandler, R., Carrive, P., 1988. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res 439, 95-106.
Bandler, R., Tork, I., 1987. Midbrain periaqueductal grey region in the cat has afferent and efferent connections with solitary tract nuclei. Neurosci Lett 74, 1-6.
Barrett, J., Cerny, F., Hirsch, J.A., Bishop, B., 1994. Control of breathing patterns and abdominal muscles during graded loads and tilt. J Appl Physiol (1985) 76, 2473-2480.
Bass, A.H., Gilland, E.H., Baker, R., 2008. Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science 321, 417-421.
Bass, A.H., Marchaterre, M.A., Baker, R., 1994. Vocal-acoustic pathways in a teleost fish. J Neurosci 14, 4025-4039.
Behbehani, M.M., 1995. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol 46, 575-605.
Beitz, A.J., 1982. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience 7, 133-159.
Blaxter, J.H., Tytler, P., 1978. Physiology and function of the swimbladder. Adv Comp Physiol Biochem 7, 311-367.
Blessing, W.W., 1997. The lower brainstem and bodily homeostasis. Oxford University Press.
Bondarenko, E., Guimaraes, D.D., Braga, V.A., Nalivaiko, E., 2016. Integrity of the dorsolateral periaqueductal grey is essential for the fight-or-flight response, but not the respiratory component of a defense reaction. Respir Physiol Neurobiol 226, 94-101.
Boyle, K.S., Riepe, S., Bolen, G., Parmentier, E., 2015. Variation in swim bladder drumming sounds from three doradid catfish species with similar sonic morphologies. J Exp Biol 218, 2881-2891.
Brainard, M.S., Doupe, A.J., 2002. What songbirds teach us about learning. Nature 417, 351-358.
Brown, J.L., 1971. An exploration study of vocalization areas in the brain of the redwinged blackbird (Agelaius phoeniceus). Behaviour 39, 91-127.
Brown, T.G., 1915. Note on the physiology of the basal ganglia and mid-brain of the anthropoid ape, especially in reference to the act of laughter. J Physiol 49, 195-207.
Brudzynski, S., 2009. Handbook of Mammalian Vocalization: An Integrative Neuroscience Approach. Academic Press.
Butler, A.B., and W. Hodos, 1996. Comparative Vertebrate Neuroanatomy. Wiley-Liss, New York.
Cameron, A.A., Khan, I.A., Westlund, K.N., Willis, W.D., 1995. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. II. Descending projections. J Comp Neurol 351, 585-601.
Celesia, G.G., 1993. Persistent vegetative state. Ann Neurol 33, 385.
Darwin, C., 1872. The expresssion of emotions in man and animals. William Clowes and Sons.
Davis, P.J., Zhang, S.P., Bandler, R., 1993. Pulmonary and upper airway afferent influences on the motor pattern of vocalization evoked by excitation of the midbrain periaqueductal gray of the cat. Brain Res 607, 61-80.
Delius, J.D., 1971. Neural substrates of vocalizations in gulls and pigeons. Exp Brain Res 12, 64-80.
Denk, D.M., Swoboda, H., Steiner, E., 1998. [Physiology of the larynx]. Radiologe 38, 63-70.
Duncker, H.R., 2004. Vertebrate lungs: structure, topography and mechanics. A comparative perspective of the progressive integration of respiratory system, locomotor apparatus and ontogenetic development. Respir Physiol Neurobiol 144, 111-124.
Dutschmann, M., Dick, T.E., 2012. Pontine mechanisms of respiratory control. Compr Physiol 2, 2443-2469.
Dutschmann, M., Herbert, H., 2006. The Kolliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci 24, 1071-1084.
Elemans, C.P., Rasmussen, J.H., Herbst, C.T., During, D.N., Zollinger, S.A., Brumm, H., Srivastava, K., Svane, N., Ding, M., Larsen, O.N., Sober, S.J., Svec, J.G., 2015. Universal mechanisms of sound production and control in birds and mammals. Nat Commun 6, 8978.
Emerson, S.B., Boyd, S.K., 1999. Mating vocalizations of female frogs: control and evolutionary mechanisms. Brain Behav Evol 53, 187-197.
Farkas, E., Jansen, A.S., Loewy, A.D., 1998. Periaqueductal gray matter input to cardiac-related sympathetic premotor neurons. Brain Res 792, 179-192.
Faunes, M., Botelho, J.F., Wild, J.M., 2017. Innervation of the syrinx of the zebra finch (Taeniopygia guttata). J Comp Neurol 525, 2847-2860.
Fine, M.L., King, T.L., Ali, H., Sidker, N., Cameron, T.M., 2016. Wall structure and material properties cause viscous damping of swimbladder sounds in the oyster toadfish Opsanus tau. Proc Biol Sci 283.
Fisher, S.E., Marcus, G.F., 2006. The eloquent ape: genes, brains and the evolution of language. Nat Rev Genet 7, 9-20.
Fitch, W.T., Reby, D., 2001. The descended larynx is not uniquely human. Proc Biol Sci 268, 1669-1675.
Garcia, M., Favaro, L., 2017. Animal vocal communication: function, structures, and production mechanisms. Curr Zool 63, 417-419.
Gargaglioni, L.H., Meier, J.T., Branco, L.G., Milsom, W.K., 2007. Role of midbrain in the control of breathing in anuran amphibians. Am J Physiol Regul Integr Comp Physiol 293, R447-457.
Gargaglioni, L.H., Milsom, W.K., 2007. Control of breathing in anuran amphibians. Comp Biochem Physiol A Mol Integr Physiol 147, 665-684.
Gerhardt, H.C., 1994. The evolution of vocalization in Frogs. Annu. Rev. Ecol. Sys. 25, 293-324.
Ghazanfar, A.A., Rendall, D., 2008. Evolution of human vocal production. Curr Biol 18, R457-460.
Goldfield, E.C., Richardson, M.J., Lee, K.G., Margetts, S., 2006. Coordination of sucking, swallowing, and breathing and oxygen saturation during early infant breast-feeding and bottle-feeding. Pediatr Res 60, 450-455.
Goodson, J.L., Bass, A.H., 2002. Vocal-acoustic circuitry and descending vocal pathways in teleost fish: convergence with terrestrial vertebrates reveals conserved traits. J Comp Neurol 448, 298-322.
Graeff, F.G., Silveira, M.C., Nogueira, R.L., Audi, E.A., Oliveira, R.M., 1993. Role of the amygdala and periaqueductal gray in anxiety and panic. Behav Brain Res 58, 123-131.
Gridi-Papp, M., 2008. The structure of vocal sounds produced with the mouth closed or with the mouth open in treefrogs. J Acoust Soc Am 123, 2895-2902.
Hammerschmidt, K., Whelan, G., Eichele, G., Fischer, J., 2015. Mice lacking the cerebral cortex develop normal song: insights into the foundations of vocal learning. Sci Rep 5, 8808.
Heckman, J.J., Proville, R., Heckman, G.J., Azarfar, A., Celikel, T., Englitz, B., 2017. High-precision spatial localization of mouse vocalizations during social interaction. Sci Rep 7, 3017.
Herbst, C.T., Stoeger, A.S., Frey, R., Lohscheller, J., Titze, I.R., Gumpenberger, M., Fitch, W.T., 2012. How low can you go? Physical production mechanism of elephant infrasonic vocalizations. Science 337, 595-599.
Hernandez-Miranda, L.R., Ruffault, P.L., Bouvier, J.C., Murray, A.J., Morin-Surun, M.P., Zampieri, N., Cholewa-Waclaw, J.B., Ey, E., Brunet, J.F., Champagnat, J., Fortin, G., Birchmeier, C., 2017. Genetic identification of a hindbrain nucleus essential for innate vocalization. Proc Natl Acad Sci U S A 114, 8095-8100.
Hofer, M.A., Shair, H.N., Brunelli, S.A., 2002. Ultrasonic vocalizations in rat and mouse pups. Curr Protoc Neurosci Chapter 8, Unit 8 14.
Holstege, G., 1989. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol 284, 242-252.
Iscoe, S., 1998. Control of abdominal muscles. Prog Neurobiol 56, 433-506.
Jurgens, U., 2009. The neural control of vocalization in mammals: a review. J Voice 23, 1-10.
Jurgens, U., Ploog, D., 1970. Cerebral representation of vocalization in the squirrel monkey. Exp Brain Res 10, 532-554.
Jurgens, U., Pratt, R., 1979. Role of the periaqueductal grey in vocal expression of emotion. Brain Res 167, 367-378.
Kang, C., Drayna, D., 2011. Genetics of speech and language disorders. Annu Rev Genomics Hum Genet 12, 145-164.
Katz, D.M., Karten, H.J., 1983. Visceral representation within the nucleus of the tractus solitarius in the pigeon, Columba livia. J Comp Neurol 218, 42-73.
Kelly, A.H., Beaton, L.E., Magoun, H.W., 1946. A midbrain mechanism for facio-vocal activity. J Neurophysiol 9, 181-189.
Kime, N.M., Ryan, M.J., Wilson, P.S., 2013. A bond graph approach to modeling the anuran vocal production system. J Acoust Soc Am 133, 4133-4144.
Kincheski, G.C., Mota-Ortiz, S.R., Pavesi, E., Canteras, N.S., Carobrez, A.P., 2012. The dorsolateral periaqueductal gray and its role in mediating fear learning to life threatening events. PLoS One 7, e50361.
King, M.S., 2007. Anatomy of the Rostral Nucleus of the Solitary Tract: in The Role of the Nucleus of the Solitary Tract in Gustatory Processing. CRC Press.
Kirzinger, A., Jurgens, U., 1985. The effects of brainstem lesions on vocalization in the squirrel monkey. Brain Res 358, 150-162.
Koutsikou, S., Watson, T.C., Crook, J.J., Leith, J.L., Lawrenson, C.L., Apps, R., Lumb, B.M., 2015. The Periaqueductal Gray Orchestrates Sensory and Motor Circuits at Multiple Levels of the Neuraxis. J Neurosci 35, 14132-14147.
Krout, K.E., Jansen, A.S., Loewy, A.D., 1998. Periaqueductal gray matter projection to the parabrachial nucleus in rat. J Comp Neurol 401, 437-454.
Kumar, V., Croxson, P.L., Simonyan, K., 2016. Structural Organization of the Laryngeal Motor Cortical Network and Its Implication for Evolution of Speech Production. J Neurosci 36, 4170-4181.
Ladich, F., 1997. Comparative analysis of swimbladder (drumming) and pectoral (stridulation) sounds in three families of catfish. Bioacoustics 8, 185-208.
Ladich, F., Winkler, H., 2017. Acoustic communication in terrestrial and aquatic vertebrates. J Exp Biol 220, 2306-2317.
Ladich F., C.S., Moller P and Kapoor B. G., 2006. Communication in Fishes. Science Publishers.
Lipkind, D., Marcus, G.F., Bemis, D.K., Sasahara, K., Jacoby, N., Takahasi, M., Suzuki, K., Feher, O., Ravbar, P., Okanoya, K., Tchernichovski, O., 2013. Stepwise acquisition of vocal combinatorial capacity in songbirds and human infants. Nature 498, 104-108.
Loyd, D.R., Murphy, A.Z., 2009. The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different? Neural Plast 2009, 462879.
Luthe, L., Hausler, U., Jurgens, U., 2000. Neuronal activity in the medulla oblongata during vocalization. A single-unit recording study in the squirrel monkey. Behav Brain Res 116, 197-210.
Magoun, H.W., Atlas, D., Ingersoll, E.H., Ranson, S.W., 1937. Associated Facial, Vocal and Respiratory Components of Emotional Expression: An Experimental Study. J Neurol Psychopathol 17, 241-255.
Marchand, J.E., Hagino, N., 1983. Afferents to the periaqueductal gray in the rat. A horseradish peroxidase study. Neuroscience 9, 95-106.
Margoliash, D., Hale, M.E., 2008. Vertebrate vocalizations. Science 321, 347-348.
Martin, J.R., 1976. Motivated behaviors elicited from hypothalamus, midbrain, and pons of the guinea pig (Cavia porcellus). J Comp Physiol Psychol 90, 1011-1034.
Meller, S.T., Dennis, B.J., 1991. Efferent projections of the periaqueductal gray in the rabbit. Neuroscience 40, 191-216.
Nakazawa, K., Shiba, K., Satoh, I., Yoshida, K., Nakajima, Y., Konno, A., 1997. Role of pulmonary afferent inputs in vocal on-switch in the cat. Neurosci Res 29, 49-54.
Newman, J.D., 1988. The Physiological Control of Mammalian Vocalization. Plenum Press, New York.
Noordzij, J.P., Ossoff, R.H., 2006. Anatomy and physiology of the larynx. Otolaryngol Clin North Am 39, 1-10.
Onodi, A., 1902. Die Anatomic und Physiologie der Kehlkoplnerven. Coblentz, Berlin.
Parmentier, M.F.a.E., 2015. Mechanisms of Fish Sound Production: in Sound communication in fishes, Friedrich Ladich ed. Springer.
Peek, F.W., Phillips, R.E., 1971. Repetitive vocalizations evoked by local electrical stimulation of avian brains. II. Anesthetized chickens (Gallus gallus). Brain Behav Evol 4, 417-438.
Penfield, H.B.W., 1922. A STUDY OF THE SHERRINGTON DECEREBRATE ANIMAL IN THE CHRONIC AS WELL AS THE ACUTE CONDITION. Brain 45, 185-265.
Potash, L.M., 1970. Vocalizations elicited by electrical brain stimulation in Coturnix coturnix japonica. Behaviour 36, 149-167.
Prakash, M., Johnny, J.C., 2015. Whats special in a child's larynx? J Pharm Bioallied Sci 7, S55-58.
Riede, T., 2011. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J Neurophysiol 106, 2580-2592.
Riede, T., Goller, F., 2010. Peripheral mechanisms for vocal production in birds - differences and similarities to human speech and singing. Brain Lang 115, 69-80.
Rohrmeier, M., Zuidema, W., Wiggins, G.A., Scharff, C., 2015. Principles of structure building in music, language and animal song. Philos Trans R Soc Lond B Biol Sci 370, 20140097.
Ryan, M.J., Guerra, M.A., 2014. The mechanism of sound production in tungara frogs and its role in sexual selection and speciation. Curr Opin Neurobiol 28, 54-59.
Saji, M., Miura, M., 1990. Thoracic expiratory motor neurons of the rat: localization and sites of origin of their premotor neurons. Brain Res 507, 247-253.
Sasaki, C.T., 2006. Anatomy and development and physiology of the larynx. GI Motility online.
Schmidt, M.F., Martin Wild, J., 2014. The respiratory-vocal system of songbirds: anatomy, physiology, and neural control. Prog Brain Res 212, 297-335.
Schmidt, M.F., McLean, J., Goller, F., 2012. Breathing and vocal control: the respiratory system as both a driver and a target of telencephalic vocal motor circuits in songbirds. Exp Physiol 97, 455-461.
Seyfarth, R.M., Cheney, D.L., 2003. Meaning and emotion in animal vocalizations. Ann N Y Acad Sci 1000, 32-55.
Seyfarth, R.M., Cheney, D.L., 2010. Production, usage, and comprehension in animal vocalizations. Brain Lang 115, 92-100.
Shiba, K., Isono, S., Nakazawa, K., 2007a. Paradoxical vocal cord motion: a review focused on multiple system atrophy. Auris Nasus Larynx 34, 443-452.
Shiba, K., Nakazawa, K., Ono, K., Umezaki, T., 2007b. Multifunctional laryngeal premotor neurons: their activities during breathing, coughing, sneezing, and swallowing. J Neurosci 27, 5156-5162.
Shiba, K., Yoshida, K., Miura, T., 1995. Functional roles of the superior laryngeal nerve afferents in electrically induced vocalization in anesthetized cats. Neurosci Res 22, 23-30.
Simonyan, K., 2014. The laryngeal motor cortex: its organization and connectivity. Curr Opin Neurobiol 28, 15-21.
Simonyan, K., Horwitz, B., 2011. Laryngeal motor cortex and control of speech in humans. Neuroscientist 17, 197-208.
Skultety, F.M., 1958. The behavioral effects of destructive lesions of the periaqueductal gray matter in adult cats. J Comp Neurol 110, 337-365.
Skultety, F.M., 1962. Experimental mutism in dogs. Arch Neurol 6, 235-241.
Subramanian, H.H., Balnave, R.J., Holstege, G., 2008. The midbrain periaqueductal gray control of respiration. J Neurosci 28, 12274-12283.
Subramanian, H.H., Holstege, G., 2009. The nucleus retroambiguus control of respiration. J Neurosci 29, 3824-3832.
Subramanian, H.H., Holstege, G., 2014. The midbrain periaqueductal gray changes the eupneic respiratory rhythm into a breathing pattern necessary for survival of the individual and of the species. Prog Brain Res 212, 351-384.
Suga, N., Schlegel, P., Shimozawa, T., Simmons, J., 1973. Orientation sounds evoked from echolocating bats by electrical stimulation of the brain. J Acoust Soc Am 54, 793-797.
Suthers, R.A., 1997. Peripheral control and lateralization of birdsong. J Neurobiol 33, 632-652.
Suthers, R.A., Fitch, W.T., Fay, R.R., Popper, A.N. , 2016. Vertebrate Sound Production and Acoustic Communication. Springer International Publishing Switzerland.
Suthers, R.A., Zollinger, S.A., 2004. Producing song: the vocal apparatus. Ann N Y Acad Sci 1016, 109-129.
Thoms, G., Jurgens, U., 1981. Role of the internal laryngeal nerve in phonation: an experimental study in the squirrel monkey. Exp Neurol 74, 187-203.
Titze, I.R., 2000. Principles of Voice Production. Prentice Hall.
Walkowiak, W., 1992. Acoustic communication in the fire-bellied toad: an integrative neurobiological approach. Ethol. Ecol. Evol. 4, 63-74.
Wetzel, D.M., Haerter, U.L., Kelley, D.B., 1985. A proposed neural pathway for vocalization in South African clawed frogs, Xenopus laevis. J Comp Physiol A 157, 749-761.
Wild, J.M., 1993a. The avian nucleus retroambigualis: a nucleus for breathing, singing and calling. Brain Res 606, 319-324.
Wild, J.M., 1993b. Descending projections of the songbird nucleus robustus archistriatalis. J Comp Neurol 338, 225-241.
Wild, J.M., 2004. Functional neuroanatomy of the sensorimotor control of singing. Ann N Y Acad Sci 1016, 438-462.
Wild, J.M., Arends, J.J., 1987. A respiratory-vocal pathway in the brainstem of the pigeon. Brain Res 407, 191-194.
Wild, J.M., Arends, J.J., Zeigler, H.P., 1990. Projections of the parabrachial nucleus in the pigeon (Columba livia). J Comp Neurol 293, 499-523.
Wild, J.M., Krutzfeldt, N.E., 2012. Trigeminal and telencephalic projections to jaw and other upper vocal tract premotor neurons in songbirds: sensorimotor circuitry for beak movements during singing. J Comp Neurol 520, 590-605.
Wild, J.M., Kubke, M.F., Mooney, R., 2009. Avian nucleus retroambigualis: cell types and projections to other respiratory-vocal nuclei in the brain of the zebra finch (Taeniopygia guttata). J Comp Neurol 512, 768-783.
Yajima, Y., Hada, J., Yoshii, N., 1976. Functional representation of ultrasonic vocalization evoked from rats by electrical stimulation of the brain. Med J Osaka Univ 27, 25-32.
Yu, F., Jiang, Q.J., Sun, X.Y., Zhang, R.W., 2015. A new case of complete primary cerebellar agenesis: clinical and imaging findings in a living patient. Brain 138, e353.
Zhang, S.P., Bandler, R., Carrive, P., 1990. Flight and immobility evoked by excitatory amino acid microinjection within distinct parts of the subtentorial midbrain periaqueductal gray of the cat. Brain Res 520, 73-82.
Zoccal, D.B., Furuya, W.I., Bassi, M., Colombari, D.S., Colombari, E., 2014. The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front Physiol 5, 238.