Parkinson's disease is a progressive age-associated neurological disorder. One of the major neuropathological hallmarks of Parkinson’s disease is the appearance of protein aggregates, mainly consisting of the protein alpha-Synuclein. These aggregates have been described both in genetic as well as idiopathic forms of the disease. Currently, Parkinson’s disease patient-specific induced pluripotent stem cells (iPSCs) are mainly used for in vitro disease modeling or for experimental cell replacement approaches. Here, we demonstrate that these cells can be used for in vivo disease modeling. We show that Parkinson’s disease patient-specific, iPSC-derived neurons carrying the LRRK2-G2019S mutation show an upregulation of alpha-Synuclein after transplantation in the mouse brain. However, further investigations indicate that the increased human alpha-Synuclein levels fail to induce spreading or aggregation in the mouse brain. We therefore conclude that grafting of these cells into the mouse brain is suitable for cell autonomous in vivo disease modeling but has strong limitations beyond that. Furthermore, our results support the hypothesis that there might be a species barrier between human to mouse concerning alpha-Synuclein spreading.
Introduction
Parkinson's disease is a progressive neurological disorder. It is the second most common neurodegenerative disease after Alzheimer’s disease. Parkinson’s disease is characterized by motor symptoms including tremor, rigidity, bradykinesia, and postural instability but also by non-motor symptoms as fatigue, depression, sleep disturbance, and dementia [1]. Although some symptomatic treatments exist, no preventive or disease-modifying therapies are currently available. The major hallmark of Parkinson’s disease is the degeneration of dopaminergic neurons in the substantia nigra of the midbrain. It is estimated that about 30 % of the familial and 3-5 % of the sporadic cases are caused by monogenetic mutations [2]. The affected genes including SNCA, PINK1, Parkin, LRRK2, DJ1, ATP13A2 and VPS35 are involved in numerous, very different biochemical processes, emphasizing a complex etiopathogenesis. Among others, cellular processes like oxidative stress, dysfunctional protein degradation and clearance, calcium dysregulation, mitochondrial dysfunction as well as protein aggregation (including prionopathy) have been implicated in the etiopathogenesis of Parkinson’s disease. Although the majority of the Parkinson’s disease cases is of idiopathic origin, the clinical symptoms and the histopathology do not differ from the genetic Parkinson’s disease cases [2]. Therefore, to study genetic cases might serve as a tool to get more insights into the complex etiopathogenesis of the pathology. A major neuropathological hallmark of Parkinson’s disease is protein aggregates that are detectable in many genetic as well as idiopathic cases. These aggregates are called Lewy bodies (LB) and Lewy neurites and consist mainly of the protein alpha-Synuclein (gene: SNCA). Several mutations in SNCA as well as increased SNCA gene doses have been previously described to cause Parkinson’s disease (reviewed in [3]). Interestingly, neuropathological studies suggest that Lewy pathology ascends from peripheral autonomic ganglia to brainstem nuclei and subsequently toward the neocortex over time [4]. The associated prionopathy hypothesis posits that conformationally altered alpha-Synuclein is transmitted between neurons and initiates protein aggregation in susceptible neurons [4]. This hypothesis is supported by the observation that alpha-Synuclein fibrils induce LB pathology in primary neuronal cultures as well as in wild-type mice [5, 6]. However, whether increased levels of alpha-Synuclein in a subset of cells would be sufficient to induce such a prion-like spreading remains unclear so far.
The reprogramming of human somatic cells into induced pluripotent stem cells (iPSCs) was a breakthrough for in vitro disease modeling [7, 8]. iPSCs resemble embryonic stem cells in all their characteristics. Numerous studies used Parkinson’s disease patient-specific iPSCs and thereof-derived neurons to gain insights into the mechanisms underlying the onset and progression of Parkinson’s disease [9]. Among other findings, it was possible to demonstrate that even mutations in Parkinson’s disease associated genes different from SNCA, can cause an upregulation of the alpha-Synuclein protein levels [10]. However, the usage of human iPSC-derived cellular models under physiological conditions, e.g. via grafting in mice, still remains unexplored.
In this study, we used an isogenic pair of Parkinson’s disease patient-specific iPSCs with the G2019S mutation in the gene LRRK2 and transplanted thereof-derived neuroepithelial stem cells (NESCs) into the striatum of mice. We demonstrated that in vivo differentiated neurons showed an upregulation of alpha-Synuclein under physiological conditions. However, we were unable to detect any spreading of alpha-Synuclein in the mouse brain. Our results suggest that human Parkinson’s disease patient-derived iPSC models were able to recapitulate key characteristics of the disease in vivo. Furthermore, they support the hypothesis that murine alpha-Synuclein might actually inhibit seeding and propagation of human alpha-Synuclein.
Material and Methods
Stem cell culture
Human induced pluripotent stem cells (iPSCs) were derived from an 81 old, female Parkinson’s disease patient carrying the LRRK2-G2019S mutation [11]. The iPSC line was gene corrected by using Zinc Finger Nucleases [11].
From these iPSCs, neuroepithelial stem cell (NESC) lines were generated and cultured as described elsewhere [12]. In brief, cells were cultured on Matrigel-coated plates in N2B27 medium (DMEM-F12 (Invitrogen)/ Neurobasal (Invitrogen) (50:50), 1:200 N2 supplement (Invitrogen,), 1:100 B27 supplement w/o Vitamin A (Invitrogen) 1:100 penicillin/streptomycin (Invitrogen), 1:100 L-Glutamine (Invitrogen) freshly supplemented with 0.5 µM Purmorphamine (Enzo Life Science), 3 µM CHIR (Axon Medchem), and 150 µM ascorbic acid (Sigma). Cells were split 1:10-1:15 once per week using Accutase. Differentiation was initiated by changing medium two days after splitting to N2B27 medium containing 1 µM PMA, 200 µM AA, 10 ng/mL BDNF (Peprotech), 10 ng/mL GDNF (Peprotech), 1 ng/mL TGF-b3 (Peprotech), and 500 µM dbcAMP (Sigma).
Transplantation
For surgeries 11-12 week old NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice were deeply anesthetized by isoflurane (4 % v/v for induction and 2 % v/v for maintenance) and bupivacain (5 mg/kg s.c.) was given as supplementary local analgesia before surgery. Additionally, buprenorphine treatment (0.1 mg/kg; s.c.;) was used as analgesia before and one day after surgery. Two weeks before transplantation, 10 µg 6-OHDA (6-Hydroxydopamine hydrobromide, Sigma Aldrich) was stereotactically injected into the striatum using following coordinates in relation to bregma: anteroposterior: 0.5 mm, mediolateral: 2.0 mm, dorsoventral: 3.5 mm (below skull). Transplantation of NESCs was performed as described before [12, 13]. In brief, NESCs (Passage 10-13) were differentiated towards dopaminergic neurons for 6 days. For transplantation, cells were dissociated to single cells with Accutase and resuspended in N2B27 medium to a concentration of 5 x 104 cells per microliter. Three microliters of the cell suspension was injected into the 6-OHDA-lesioned striatum using a Hamilton 7005KH 5-µl syringe (LRRK2-G2019S n=6, LRRK2-WT n=3).From these iPSC
Perfusion, sectioning, and immunohistochemical analysis
Perfusion, sectioning, and immunohistochemical analysis were performed 11 weeks post-transplantation as described previously [12, 13]. The following primary antibodies were used: human Nuclei (mouse, 1:200, Millipore), human NCAM (mouse, 1:100, Santa Cruz), and alpha-Synuclein (rabbit, 1:600, Sigma). Alexa fluorophore-conjugated secondary antibodies (Invitrogen) and Hoechst 33342 (Invitrogen) were used to visualize primary antibodies and nuclei, respectively. Sections (40 µm) from the center of the graft were evaluated using a Zeiss LSM 710 confocal microscope. 3D surface structures of the z-stacks taken by the confocal microscope were created using IMARIS software (bitplane). For this, three different ROIs (graft, proximal to graft and distal to graft) were chosen and the values of the threshold (background substraction) were automatically set. The endogenous mouse stainings of the striatal myelinated fibers were not taken into consideration for the analysis.
Data and statistical analysis
The volume, the mean intensity, and maximum intensity of every created surface were defined by Imaris software. The average or the sum of the different parameters was calculated for each animal. Ratios of the different ROIs were created as indicated and t-tests were performed using SigmaPlot. n specifies the number of mice and statistical significance is considered to be P < 0.05. Prism 6.01 (GraphPad) was used for data illustration and data are presented as mean + SEM.
Results
Parkinson’s disease-specific neurons, expressing LRRK2-G2019S, upregulated alpha-Synuclein in vivo
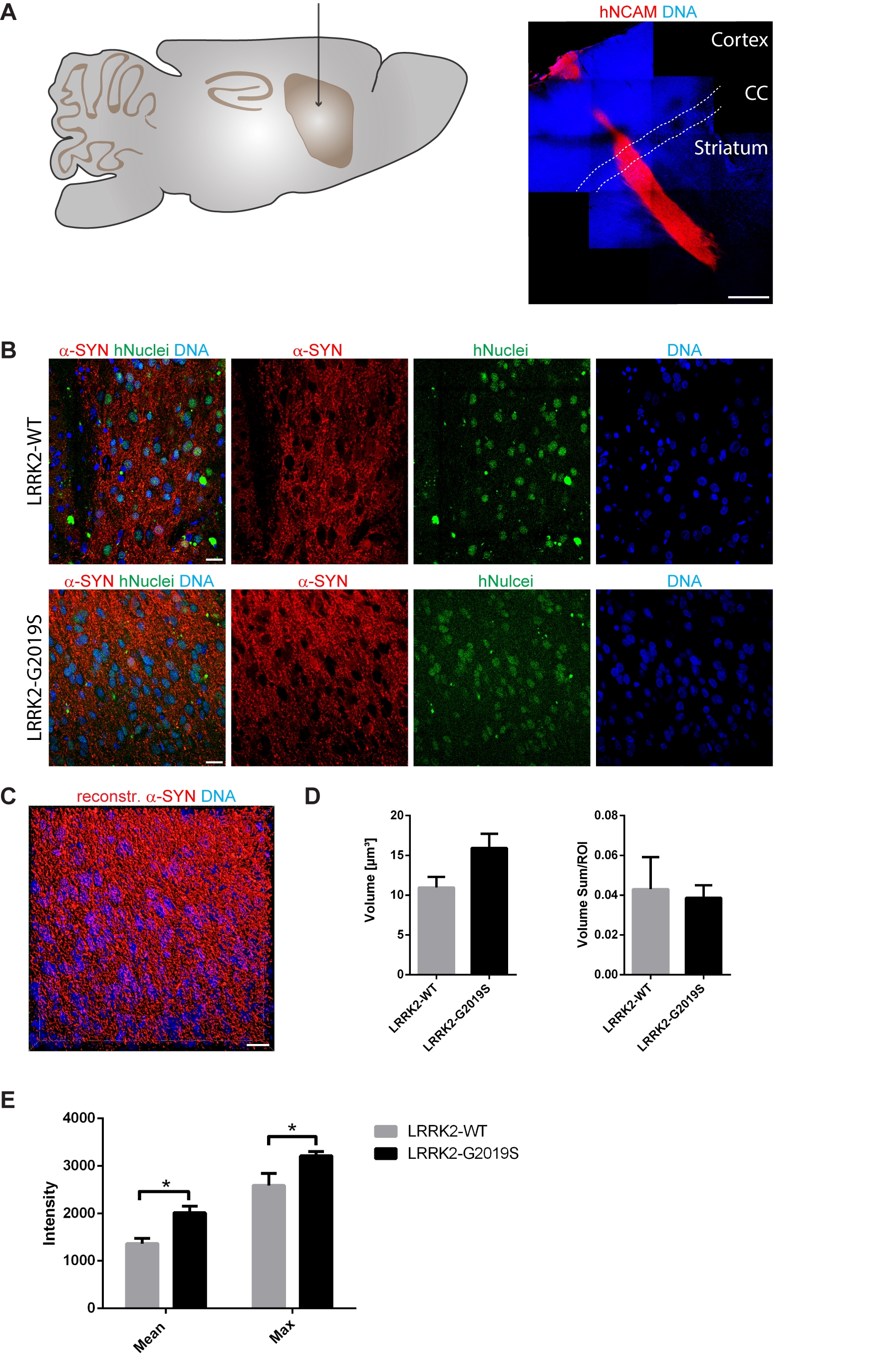
Previously, it has been shown in vitro that human neurons expressing the Parkinson’s disease-associated G2019S mutation in the LRRK2 gene have elevated levels of alpha-Synuclein [10]. As a first step, we were aiming at determining whether this is also the case in vivo. Accordingly, we transplanted 1,5 x 105 human iPSC-derived NESCs that were pre-differentiated for the neuronal lineage (for details see Materials and Methods section), into the striatum of 11-12 week old NOD.SCID mice. In order to investigate the impact of the LRRK2-G2019S mutation, we used patient-derived cells expressing this mutation as well as a corresponding isogenic line where the mutation has been corrected. Human cells were distinguished from the surrounding mouse cells with human-specific antibodies against NCAM and human nuclei (Fig. 1A, B). 11 weeks after transplantation in the mouse striatum, we saw robust survival and neuronal differentiation for both lines as indicated with the anti-human-NCAM antibody (Fig. 1A). In order to detect the levels of alpha-Synuclein in the grafted cells, we stained with anti-alpha-Synuclein-specific antibodies (Fig. 1B). For quantification, we reconstructed the surfaces of the alpha-Synuclein signal in the grafts with IMARIS (Fig. 1C). The volume and the intensity of the alpha-Synuclein signal was quantified from the reconstruction (Fig. 1D, E). This quantification revealed that expression of LRRK-G2019S led to a negligible increase in the mean volume of the alpha-Synuclein signal (Fig. 1D). However, the mean and the maximum intensity of the alpha-Synuclein signal was significantly increased upon expression of the Parkinson’s disease-associated LRRK2-G2019S mutation (Fig. 1E). These results indicate that human neurons carrying the LRRK2-G2019S mutation express higher levels of alpha-Synuclein under physiological conditions.
Figure 1. LRRK2-G2019S mutation led to a higher expression of alpha-Synuclein in grafted neurons. A Schematic overview of the adult mouse brain (left) representing the stereotactic target side of the striatum for the transplantation. 11 weeks after transplantation, the species-specific antibody hNCAM revealed a sound survival and neuronal differentiation of the graft (right). Dashed lines indicate the corpus callosum. B Immunohistological stainings indicated the expression of alpha-Synuclein in both cell lines after transplantation. C Representative 3D surface reconstruction of alpha-Synuclein of the graft shown in the lower panel of B. D Quantification of the volume and volume sum/region of interest of the reconstructed alpha-Synuclein surfaces. E Quantification of the mean and maximum intensity of the reconstructed alpha-Synuclein surfaces. Scale bars: 500 µm (A) and 20 µm (B and C). Error bars represent mean + SEM (LRRK2-WT n=3, LRRK2-G2019S n=6); α-SYN: alpha-Synuclein, CC: corpus callosum, max: maximum, ROI: region of interest.
Grafted neurons did not induce alpha-Synuclein spreading in the mouse brain
Parkinson’s disease is a progressive disorder characterized by the spreading of protein aggregates, mainly consisting of alpha-Synuclein. It has been hypothesized that this appearance of aggregates spreads in a prion-like fashion [4]. Aggregated alpha-Synuclein has the ability to induce further aggregation of soluble alpha-Synuclein. Hence, a seed of alpha-Synuclein aggregation would be sufficient to start the process.
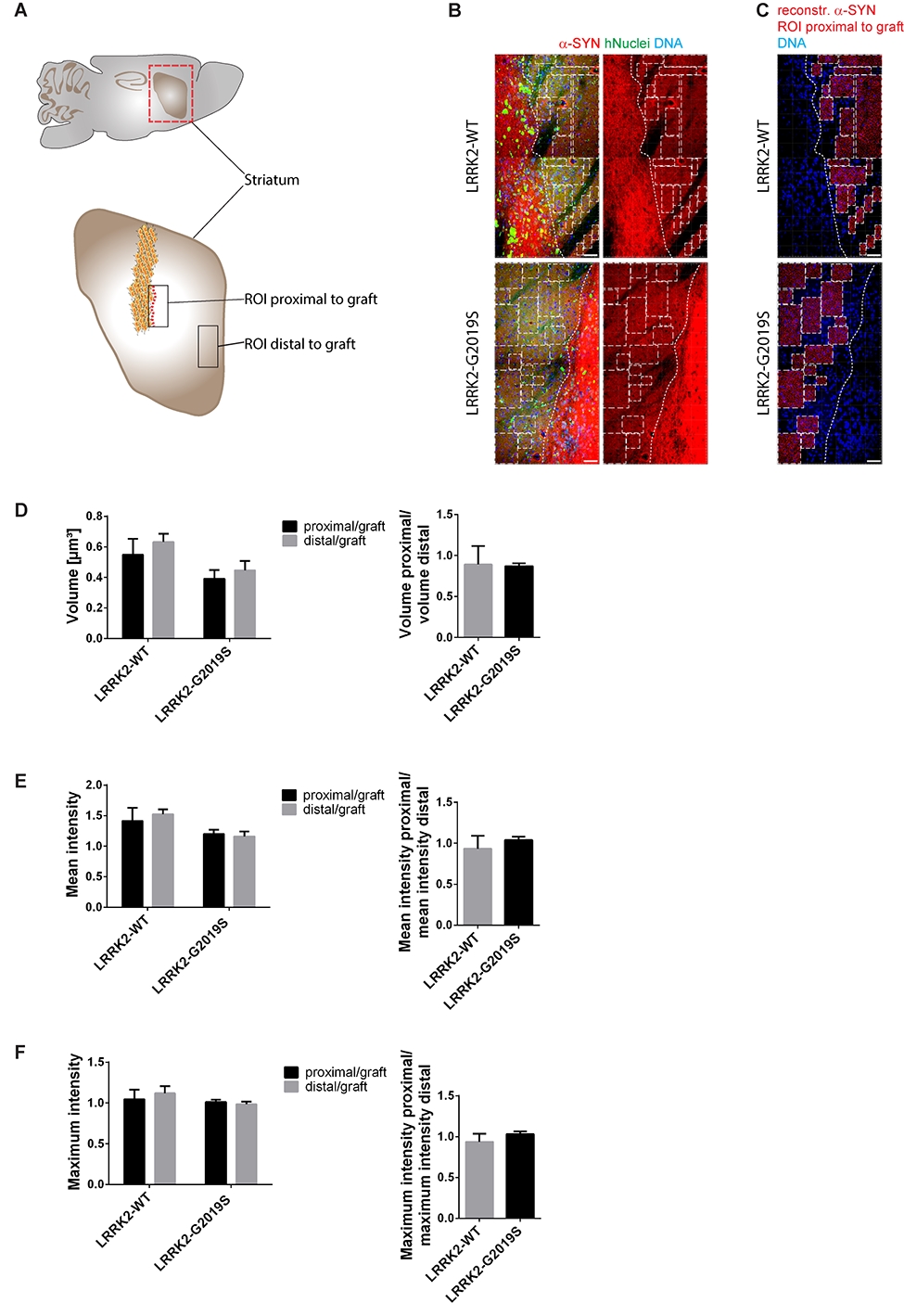
In order to test whether our in vivo phenotyping approach would be able to recapitulate this spreading process, we analyzed the levels of endogenous mouse alpha-Synuclein in a region close (proximal) to the graft in comparison to a region far (distal) from the graft (Fig. 2A). Accordingly, a 3D surface reconstruction of the endogenous mouse alpha-Synuclein signal was performed with IMARIS (Fig. 2B and C). If alpha-Synuclein indeed had spread from the graft into the surrounding tissue, we would have expected to find significant differences in the volume (Fig. 2D) or intensity (Fig. 2E, F) of the alpha-Synuclein signal in a region proximal to the graft compared to a region distal to the graft when normalizing both regions to the graft.Particularly, we expected to see such differences in cases where cells with the LRRK2-G2019S mutation have been transplanted. Moreover, we compared the ratio of the two regions between the different genotypes. However, in none of the investigated parameters significant differences were detectable (Fig. 2D-F). We therefore conclude that in the here chosen paradigm Parkinson’s disease-associated alpha-Synuclein spreading is not detectable.
Discussion
The spreading of alpha-Synuclein protein aggregates, in a prion-like fashion, is believed to be the underlying mechanism for the progression of Parkinson’s disease pathology through the human brain [4]. Furthermore, somatic mutations occurring during embryogenesis can lead to genetic mosaicism in the brain. Typically, genotyping is conducted in mesoderm-derived lymphocytes. Therefore, mutations in ectoderm-derived neural cells will be missed [14]. Consequently, it is conceivable that Parkinson’s disease patients that are classified as idiopathic, actually carry a Parkinson’s disease-associated mutation in a subset of neurons. In fact, somatic mutations have been previously associated to neurodegenerative disorders. It was suggested that differences in Parkinson’s disease phenotype in monozygotic twins with LRRK2 mutations are caused by additional somatic mutations [15, 16]. In the past, addressing the hypothesis of prion-like spreading of alpha-Synuclein and genetic mosaicism as potential source for aggregates of alpha-Synuclein was complicated because of the lack of appropriate models. In this study, we show that Parkinson’s disease patient-specific iPSC-derived neurons upregulated alpha-Synuclein after transplantation which is believed to be a first important step to induce protein aggregation and spreading. Therefore, we conclude that this approach can be used for in vivo disease modeling. However, we further demonstrate that this alpha-Synuclein failed to spread in the mouse brain.
At first glance, it seems surprising not to see alpha-Synuclein spreading, because previous studies in vitro [5, 17] as well as in vivo [6, 18] have shown spreading. Furthermore, our finding that Parkinson’s disease-specific cells with the LRRK2-G2019S mutation showed higher levels of alpha-Synuclein, supports their usability for modeling Parkinson's disease in vivo and indicates that they in principle might be able to induce alpha-Synuclein aggregation and spreading. However, it is noticeable that all these studies did not use a cross-species approach. Either human alpha-Synuclein spreading was investigated in human cells or in mice engineered to express human alpha-Synuclein. On the other hand, mouse alpha-Synuclein was tested in mouse cells or mice in vivo. Interestingly, a recent study using a cross-species approach reported that the mouse alpha-Synuclein protein significantly attenuated the formation of aggregates of human-alpha-Synuclein [19]. In other words, the mouse alpha-Synuclein inhibited the aggregation of the human form, which implies the existence of a species barrier. This finding is also supported by a report showing that mouse alpha-Synuclein inhibited the fibrillization of human alpha-Synuclein in vitro [20]. In this context, it is further interesting to note that the A53T mutation in human alpha-Synuclein causes Parkinson’s disease while the mouse version of alpha-Synuclein naturally expresses a Threonine at position 53. However, based on the here obtained results we cannot rule out that the failure to see alpha-Synuclein spreading in the mouse brain could be due to initially too low levels of human alpha-Synuclein in the transplanted cells. Additionally, we cannot exclude that an even longer duration of the experiment would have led to mouse alpha-Synuclein upregulation, aggregation, and spreading. Finally, the depletion of dopaminergic neurons in the striatum with 6-OHDA, preceding the transplantation, might have had a negative impact on the potential spreading.
Figure 2. Alpha-Synuclein spreading from the graft was not detected in the surrounding tissue. A Schematic overview of the mouse adult brain indicating the ROIs that were chosen to analyse endogenous mouse alpha-Synuclein close (proximal) as well as far (distal) from the graft. B Representative images of the ROIs that were chosen proximal to the graft. Dashed lines define the edges of the graft. Squares mark the ROIs proximal to the graft that were used to create 3D surfaces shown in C. C 3D surface reconstruction of endogenous mouse alpha-Synuclein of the ROIs shown in B. D-F Quantification of the volume, the mean intensity and the maximum intensity of the reconstructed endogenous mouse alpha-Synuclein surfaces proximal to the graft and distal to the graft. Left: Proximal and distal ROIs of endogenous mouse alpha-Synuclein were normalized to the ROIs of the graft and eventually the different distances were compared to each other within the same cell line. Right: The ratio of the different regions of endogenous mouse alpha-Synuclein were compared between the different cell lines. Scale bars: 50 µm (B and C). Error bars represent mean + SEM (LRRK2-WT n=3, LRRK2-G2019S n=6); α-SYN: alpha-Synuclein, max: maximum, ROI: region of interest.
Conclusion
Overall these results clearly indicate that it is of critical importance to choose the appropriate model and tools to study alpha-Synuclein aggregation and spreading. In particular, new human-specific organoid models recapitulating essential features of the human midbrain [21, 22] might represent interesting alternatives for the future. These models will allow combining the advantages of tissue-like specimens (complex system, in vivo, cellular heterogeneity) with high relevance for the human situation, particularly when they are derived from human Parkinson’s disease patient-specific stem cells.
Abbreviations: α-SYN: alpha-Synuclein, CC: corpus callosum, iPSCs: induced pluripotent stem cells, LB: Lewy bodies, max: maximum, NESCs: neuroepithelial stem cells, ROI: region of interest SNCA: Synuclein Alpha
Ethical approval and consent to participate
All work with human iPSCs and thereof derived cells has been approved by the Ethics Review Panel (ERP) of the University Luxembourg as well as by the Luxembourgish Comité National d'Ethique de Recherche (CNER). The CNER reference number is 201305/04
All animal experimentation was approved by the appropriate Luxembourg governmental agencies (Ministry of Health and Ministry of Agriculture).
Consent for publication
Not applicable
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KH, SB, and JCS contributed to the conception and design of the study. KH and LMS performed experiments and analyzed data. JCS drafted the manuscript and KH prepared main figures. All authors read and approved the final manuscript.
| Attachment | Size |
|---|---|
| 4.93 MB |
The JCS lab is supported by the Fonds National de la Recherche (FNR) (CORE, C13/BM/5791363). This is an EU Joint Programme. – Neurodegenerative Disease Research (JPND) project (INTER/JPND/14/02; INTER/JPND/15/11092422). Further support comes from the SysMedPD project which has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 668738. KH received financial support from a private philanthropist as well as from the Fondation du Pélican de Mie et Pierre Hippert-Faber. LMS is supported by a fellowship from the FNR (AFR, Aides à la Formation-Recherche).
The authors would like to thank Inga Brüggemann and Thea van Wüllen for excellent technical assistance. We further acknowledge support through the pluripotent stem cell facility at the LCSB.
Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY: How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 2012, 135(Pt 6):1860-1870.
Klein C, Westenberger A: Genetics of Parkinson's disease. Cold Spring Harb Perspect Med 2012, 2(1):a008888.
Kalinderi K, Bostantjopoulou S, Fidani L: The genetic background of Parkinson's disease: current progress and future prospects. Acta Neurol Scand 2016, 134(5):314-326.
Braak H, Del Tredici K: Neuroanatomy and pathology of sporadic Parkinson's disease. Adv Anat Embryol Cell Biol 2009, 201:1-119.
Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM: Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 2011, 72(1):57-71.
Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, Lee VM: Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 2012, 338(6109):949-953.
Takahashi K, Yamanaka S: Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126(4):663-676.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R et al: Induced pluripotent stem cell lines derived from human somatic cells. Science (New York, NY) 2007, 318(5858):1917-1920.
Hillje AL, Schwamborn JC: Utilization of stem cells to model Parkinson's disease – current state and future challenges. Future Neurology 2016, 11(2):171-186.
Sanchez-Danes A, Richaud-Patin Y, Carballo-Carbajal I, Jimenez-Delgado S, Caig C, Mora S, Di Guglielmo C, Ezquerra M, Patel B, Giralt A et al: Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson's disease. EMBO Mol Med 2012, 4(5):380-395.
Reinhardt P, Schmid B, Burbulla LF, Schondorf DC, Wagner L, Glatza M, Hoing S, Hargus G, Heck SA, Dhingra A et al: Genetic correction of a LRRK2 mutation in human iPSCs links parkinsonian neurodegeneration to ERK-dependent changes in gene expression. Cell stem cell 2013, 12(3):354-367.
Reinhardt P, Glatza M, Hemmer K, Tsytsyura Y, Thiel CS, Hoing S, Moritz S, Parga JA, Wagner L, Bruder JM et al: Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PloS one 2013, 8:e59252.
Hemmer K, Zhang M, van Wullen T, Sakalem M, Tapia N, Baumuratov A, Kaltschmidt C, Kaltschmidt B, Scholer HR, Zhang W et al: Induced neural stem cells achieve long-term survival and functional integration in the adult mouse brain. Stem cell reports 2014, 3(3):423-431.
Proukakis C, Houlden H, Schapira AH: Somatic alpha-synuclein mutations in Parkinson's disease: Hypothesis and preliminary data. Movement Disorders 2013, 28:705-712.
Van Broeckhoven C: The future of genetic research on neurodegeneration. Nat Med 2010, 16(11):1215-1217.
Schneider SA, Johnson MR: Monozygotic twins with LRRK2 mutations: Genetically identical but phenotypically discordant. Movement Disorders 2012, 27:1203-1204.
Luk KC, Song C, Brien PO, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM: Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. PNAS 2009, 106.
Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VM: Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. The Journal of experimental medicine 2012, 209(5):975-986.
Fares MB, Maco B, Oueslati A, Rockenstein E, Ninkina N, Buchman VL, Masliah E, Lashuel HA: Induction of de novo alpha-synuclein fibrillization in a neuronal model for Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America 2016, 113(7):E912-921.
Rochet JC, Conway KA, Lansbury PT, Jr.: Inhibition of fibrillization and accumulation of prefibrillar oligomers in mixtures of human and mouse alpha-synuclein. Biochemistry 2000, 39(35):10619-10626.
Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Goke J, Tan ZY, Saw TY, Tan CP, Lokman H et al: Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell stem cell 2016, 19(2):248-257.
Monzel AS, Smits LM, Hemmer K, Hachi S, E. LM, van Wuellen T, Jarazo J, Walter J, Brüggemann I, Boussaad I et al: Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem cell reports 2017, 8.