The aim of the present study was to explore the effects of 8-OH-DPAT, 5-HT1A receptor agonist and NAN-190, 5-HT1A receptor antagonist on anxiety-related behavior in the adult gonadectomized (GDX) male rats. Moreover, another goal of this work was to investigate whether the combination of 8-OH-DPAT or NAN-190 plus testosterone propionate (TP) could affect anxiety-like behavior more than TP alone in the adult GDX rats. Two weeks after gonadectomy, GDX rats were subjected by treatments with the solvent, TP (0.5 mg/kg, s.c.), 8-OH-DPAT (0.05 mg/kg, s.c.), NAN-190 (0.1 mg/kg, i.p.), 8-OH-DPAT in a combination with TP or NAN-190 in a combination with TP during 14 days. Experimental groups of GDX rats and control group of intact males were then tested in the elevated plus maze (EPM) and the open field test. 8-OH-DPAT treatment failed to modify anxiety-like behavior of GDX rats in the EPM as compared to the GDX rats given with oil solvent. NAN-190 injected alone or in combination with TP to GDX rats resulted in a significant anxiolytic-like effect as compared to the GDX given with oil solvent or TP application. Our data indicate that the combination of NAN-190 and TP is more effective than TP alone in GDX rats inducing a more profound anxiolytic-like effect in the EPM. Thus, the results of this study suggest that effects of 5-HT1A receptor agonist/antagonist can modify anxiety level in opposite direction in male rats after gonadectomy.
Introduction
Anxiety disorders are the most frequent psychiatric conditions among the affective-related diseases (Davidson, 2000). Lifetime prevalence of anxiety disorders is reportedly as high as 31%; higher than the lifetime prevalence of depression disorders and substance use disorders (Wittchen, 2002; Somers et al., 2006; Kessler et al., 2007). Moreover, studies report that about 40% of patients diagnosed with anxiety and related disorder are untreated (Kronke et al., 2007; Martin-Merino et al., 2010).
The role of serotonin (5-HT) in anxiety disorders is now well established and it has been conclusively shown that increase in central serotonergic activity invariably leads to anxiety, whereas a decrease in the brain 5-HT activity results in anxiolysis (Matsudaa, 2013). Among the different 5-HT receptor subtypes, the 5-HT1A receptors have received a great deal of attention mainly because it is implicated in anxiety and depression (Lucki et al., 1994; De Vry, 1995; Barnes and Sharp, 1999; Blier and Ward, 2003 ). 5-HT1A receptors are located both pre- and postsynaptically (Albert et al., 2014). Presynaptic 5-HT1Areceptors (as somadendritic 5-HT1A autoreceptors) are present on serotonergic neurons in the dorsal and medial raphe nuclei, and postsynaptic 5-HT1A receptors are found at high density in the limbic regions and in the frontal and entorhinal cortices (Pazos and Palacios, 1985; Vergé et al., 1986). There are differences in the G-protein coupling between pre- and postsynaptic 5-HT1A receptors. The effects of 5-HT1A receptor agonists/antagonists on behavioral processes have been extensively studied (De Vry, 1995; Barnes and Sharp, 1999; Blier and Ward, 2003; Martin-Merino et al., 2010). Studies in animal models suggested that the 5-HT1A receptor is a potential target for the treatment of anxiety, depression, pain, and drug dependence (Albert and Le Francois, 2010; Celada et al., 2013).
Hormonal mechanisms, especially the dysregulation of the hypothalamic-pituitary-gonadal (HPG) axis, underlying mood and stress-related disorders have gained renewed great interest (Altemus, 2006; Marshall, 2011). Nowdays, the significance of gonadal hormones in the development of mood disorders has been extensively investigated (Watson et al., 2002; Young and Korszun, 2002; Swaab et al., 2005). Androgens, testosterone and its active metabolite, have several important actions in androgen responsive tissue throughout the body (Edinger and Frye, 2005, 2006; Marshall, 2011; Hogervorst, 2013). In addition, the classic roles of androgens in development and sexual differentiation, androgens also have several beneficial actions in the brain. Androgens have been shown to affect brain regions known to be involved in the modulation of mood and affective-related behavior (Frye and Seliga, 2001; Frye and Edinger, 2004; Frye et al., 2008; Hodosy et al., 2012). Testosterone and its metabolites have been shown to possess anxiolytic-like effects in several animal models of anxiety, reducing anxiety-like behavior in male rodents (Kronke et al., 2007; Khakpai, 2014). Moreover, testosterone replacement in castrated males ameliorates anxiety-like behavior (McDermott et al., 2012; McHenry et al., 2014). These data indicate a close relationship between anxiety-related disorders and low testosterone level.
On the other hand, there is evidence that activity of 5-HT system and 5-HT1A receptors in the brain are influenced by androgens (Marshall, 2011; Garcia-Garciaa et al., 2014). Androgens deficiency induced by gonadectomy modified 5-HT1A receptors binding and/or their expression throughout the brain, and these effects were reversed by testosterone propionate replacement (Zhang et al., 1999). Moreover, androgens changed sensitivity of 5-HT1A receptors and 5-HT transporter protein metabolism in the central nervous system (Frye and Seliga, 2001; Fernandez-Guasti and Martinez-Mota, 2003, 2005; Frye and Edinger, 2004; Edinger and Frye, 2005, 2006; Frye et al., 2008). Thus, gonadal hormone and 5-HT systems have been shown to interact with each other, resulting in modulation of each other’s expression and function.
Based on evidence of 5-HT system and androgens involvement in the mechanisms of anxiety-related behavior, we hypothesized that stimulation or blockade of 5-HT1A receptors would modify anxiety-like behavior of male rats with different levels of gonadal steroids. As the rule the typical anxiolytics are effective and well tolerated in all anxiety disorders, however, several weeks are required until there is an onset of action (Martinez-Conde et al., 1985; Bandelow et al., 2008). Since a lot of well-known anxiolytic must be taken repeatedly before their therapeutic efficacy becomes apparent (Harto et al., 1988; Glitz and Pohl, 1991). Moreover, the effects of acute and repeated treatments with 5-HT1A receptor agonist/antagonist in different animal models of anxiety-like behavior are different and opposite in non-castrated male subjects. This is consistent with the view that acute administration of 5-HT1A receptor ligands stimulates 5-HT system, whereas continuous treatment desensitizes it (Matsudaa, 2013; Garcia-Garciaa et al., 2014).
The main aim of the present study was to determine if repeated systemic treatment with 8-OH-DPAT, 5-HT1A receptor agonist and NAN-190, 5-HT1A receptor antagonist affected on anxiety-related behavior in the adult gonadectomized (GDX) male rats. In this study, a repeated treatment with 5-HT1A receptor agonist/antagonist was only used since following repeated application of these 5-HT receptor ligands an adaptation of the 5-HT system may be produced (Xu et al., 1997; Sheehan and Sheeh, 2007). Moreover, it is interesting to clarify whether after repeated treatment of 5-HT1A receptor ligands their effects on anxiety-like behavior may be determined and depended from the hormonal state of male rats (low testosterone level or testosterone application). Therefore, another aim of this work was to investigate whether repeated combined treatment with 8-OH-DPAT or NAN-190 plus testosterone propionate (TP) could affect anxiety-like behavior more than TP alone in the adult GDX rats.
Materials and Methods
Animals
The study used 140 adult male rats of Wistar strain (purchased from Rappolovo, Russia) weighing 180-200 g at the start of the experiment. For at least a week prior to the experiment, the rats were housed six to a cage under standard environmental conditions: constant temperature of 23 ± 1°C, 60% humidity, 12-h light/dark cycle (light on at 8:00 a.m.), food and water ad libitum. All animals were gently handled by experienced animal facility staff each day for a week prior to experimental procedures. Any environmental or physical stress was avoided in order to habituate the rats to manipulation. Animals were randomly assigned to experimental groups and were used only once in the behavioral experiments. The behavioral tests were conducted between 09:00 a.m. and 01:00 p.m. Experiments were carried out in a soundproof and air-regulated experimental room, to which animals were habituated at least 30 min before each test. All experiments were carried out in accordance with the Guide for Care and Use of Laboratory Animals, published by the National Institute of Health (National Research council, publication no. 85-23, revised in 1996), and the Animal Welfare Assurance Renewal for Pavlov Institute of Physiology. The rationale, design, and methods of this study were approved by the Ethical Committee for Animal Research, Pavlov Institute of Physiology.
Gonadectomy
The male rats were anesthetized with a mixture of ketamine/xylazine (ketamine: 70 mg/kg b.w. and xylazine: 10 mg/kg b.w., i.p.) and bupivacaine (0.25% solution: 0.4 ml/kg b.w.) was applied topically as analgesic. The non-steroidal anti-inflammatory drug meloxicam (1 mg/kg b.w.) was injected subcutaneously. Following disinfection of the skin (with alcohol and betadine), a 1-2 cm ventral midline incision was made in the scrotum of adult male rats to expose the tunica. The tunica was pierced and both testes were extracted to expose the underlying blood vessels, which were ligated with silk suture. The testes were excised and all vessels and ducts were placed back into the tunica prior to suturing. The effectiveness of castration or exogenous administration of TP were determined after sacrifice, and bulbospongiosus muscles were dissected from the penile bulb and immediately weighed. Sham-operated animals received incisions with no testicle removal. Following gonadectomy, GDX males were placed in a community cage with free access to food and water. GDX male rats were allowed to have 14 days for postoperative recovery before administration of oil solvent, 8-OH-DPAT, NAN-190 or TP.
Drugs
5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino) tetralin – 8-OH-DPAT (H-140, Sigma Chemical Co, USA) was dissolved in sterile saline (0.9%). 5-HT1A receptor antagonist [1-(2-methoxyphenyl)-4-[4-(2-phthalimido)butyl]-piperazine hydrobromide] – NAN-190 (N-3529, Sigma Chemical Co, USA) was dissolved in a vehicle of propilenglycol (40%), ethyl alcohol (10%) and sterile distilled water (50%). The testosterone propionate, TP (T-1875, Sigma Chemical Co, USA) was dissolved in sterile sesame oil. All solutions were freshly prepared before each experimental series. All males were 3.5 months old at the onset of pharmacological treatments.
Experimental groups
Two weeks after gonadectomy, GDX male rats were randomly assigned to each of the experimental groups and subjected to the different treatments. All male GDX and intact rats were divided into 7 groups (n=10 per group) for each behavioral tests. The first group consisted of sham-operated intact male rats (control) treated with saline daily (control/solvent). The two other groups were of intact sham-operated male rats treated with 8-OH-DPAT at a daily dose of 0.05 mg/kg, s.c. (intact/8-OH-DPAT) and intact sham-operated male rats treated with NAN-190 at a daily dose of 0.1 mg/kg, i.p. (intact/NAN-190). The next groups were of GDX male rats received the oil solvent daily (GDX/solvent), GDX rats treated with TP at a daily dose of 0.5 mg/kg, s.c. (GDX/TP). The other groups consisted of GDX male rats treated with 8-OH-DPAT (GDX/8-OH-DPAT), GDX males treated with NAN-190 (GDX/NAN-190), GDX rats treated with 8-OH-DPAT daily in combination with TP (GDX/8-OH-DPAT/TP), GDX males with NAN-190 daily in combination with TP (GDX/NAN-190/TP). All experimental groups are presented in the Table 1.
Two weeks after gonadectomy, 8-OH-DPAT, NAN-190, TP or oil solvent were administered for 14 days once daily before the behavioral tests. Similarly, 8-Oh-DPAT, NAN-190 or saline were also chronically injected daily for 14 days into the intact rats. One hour after the last injection, testing in the elevated plus maze (EPM) and the open field test (OFT) was carried out as described below. During all behavioral tests, the control and experimental groups of rats were also treated with 8-OH-DPAT, NAN-190, TP, saline or oil solvent.
8-OH-DPAT or NAN-190 were injected 30 min prior to TP treatment when it was co-administration of these pharmacological substances. 5-HT1A receptor agonist/antagonist alone or in combination with TP were chronically injected for 14 days. The last injection of preparation being made 1 h prior to behavioral testing. 8-OH-DPAT, NAN-190, saline, sesame oil or TP were injected in in a volume of 0.1 ml. The dose of TP (0.5 mg/kg, s.c.) was chosen from our previous studies performed by Fedotova (2014, 2015). In this study, the dose of TP was used because previous study has shown this dose to be sufficient and effective for behavioral outcomes and can be as trigger dose for reducing of behavioral impairments after gonadectomy. However, this dose of TP is not sufficient to maintain physiological level of testosterone similarly to non-castrated male rats, since the aim of this work was to investigate whether repeated combined treatment with 8-OH-DPAT or NAN-190 plus testosterone propionate (TP) in a low dose could affect anxiety-like behavior more than TP alone in the adult GDX rats. Thus, used dose of TP in the present study is a minimal behavioral dose. The doses of 8-OH-DPAT and NAN-190 were chosen accordingly the studies performed by Fedotova and co-workers (2004).
Behavioral tests
Before testing, animals were handled daily for 1 week. Behavioral experiments were carried out in a soundproof and air-regulated experimental room, to which animals were habituated, at least 30 min before each test. Any environmental or physical stress were avoided in order to habituate the rats to manipulation for behavioral tests. The apparatus used in all behavioral experiments were thoroughly cleaned after each test session with a cleaning solution from Vekton (Russia, with a composition of ammonia 0.5%, ethanol 15%, extran 10%, isopropyl alcohol 5%, citrus aromatizing 19%, and distilled water 50.5% as v/v%).
Elevated plus maze
To investigate the changes in anxiety-like behavior, control intact rats and all experimental groups of GDX male rats were subjected to the elevated plus maze test (EPM) (Fedotova et al., 2004). EPM is a widely used test of anxiety-like behavior and was used to assess an anxiety-like behavioral responses (Pellow et al., 1985). This test is sensitive to putative anxiogenic-like and anxiolytic-like drugs (Menzaghi et al., 1994).
It is designed to present the animal with a conflict between its natural tendency to explore a novel environment and its reluctance to move away from the sheltering walls and into the open environment in which the risk of falling or exposure to predators is much higher. The maze was made of grey Plexiglas and consisted of four arms (50 cm long and 10 cm wide); two arms had 40-cm-high dark walls (closed arms), and two arms had 0.5-cm-high ledges (open arms). In the center of the arms of EPM located cross-wise there was an open area in the size of 10 × 10 cm. The floor of the apparatus was 50 cm high. The experimental room was lit by a 60 Watt bulb placed 1.75 m above the central square of the maze (22 lx in the maze central square). For testing, rats were placed individually into the center of the maze facing a closed arm and removed after a 5-min period. The number of entrances and the time spent into the open or closed arms were registered during time of testing. A video camera was installed above the cage to record the activity of the rats. Two independent observers measured the behavioral variables. After each test session, the EPM apparatus was carefully cleaned and deodorized with the Vekton cleaning solution.
Open field test
To investigate the changes in spontaneous locomotor activity, rearing, and grooming behavior, all experimental groups of offspring were submitted to a 5-min period to the open field test (OFT). The OFT was consisted of a square platform (80.0 cm × 80.0 cm; wall height 36.0 cm) (De Vry, 1995). The floor of the platform was divided into 16 equal squares of 19.5 cm × 19.5 cm. The platform was illuminated by a light source (lamp 60 W). Two independent observers (blind to treatment groups) measured the behavioral variables. A video camera was installed above the cage to record the activity of the rats. After each test session, the OFT apparatus was carefully cleaned and deodorized with the Vekton cleaning solution.
Statistical analysis
All values were expressed as mean ± S.E.M. Comparisons between values were performed using two-way ANOVA test with between-subject factors of hormone conditions (GDX or GDX plus TP) and drug treatment (8-OH-DPAT or NAN-190) followed by Dunnett’s test for multiple comparisons post-hoc test. Statistical analysis was performed using SPSS version 11.5. Differences with p<0.05 were considered significant.
Results
Effects of 8-OH-DPAT and NAN-190 on anxiety-like behavior of GDX males and GDX males treated with testosterone propionate in the elevated plus maze
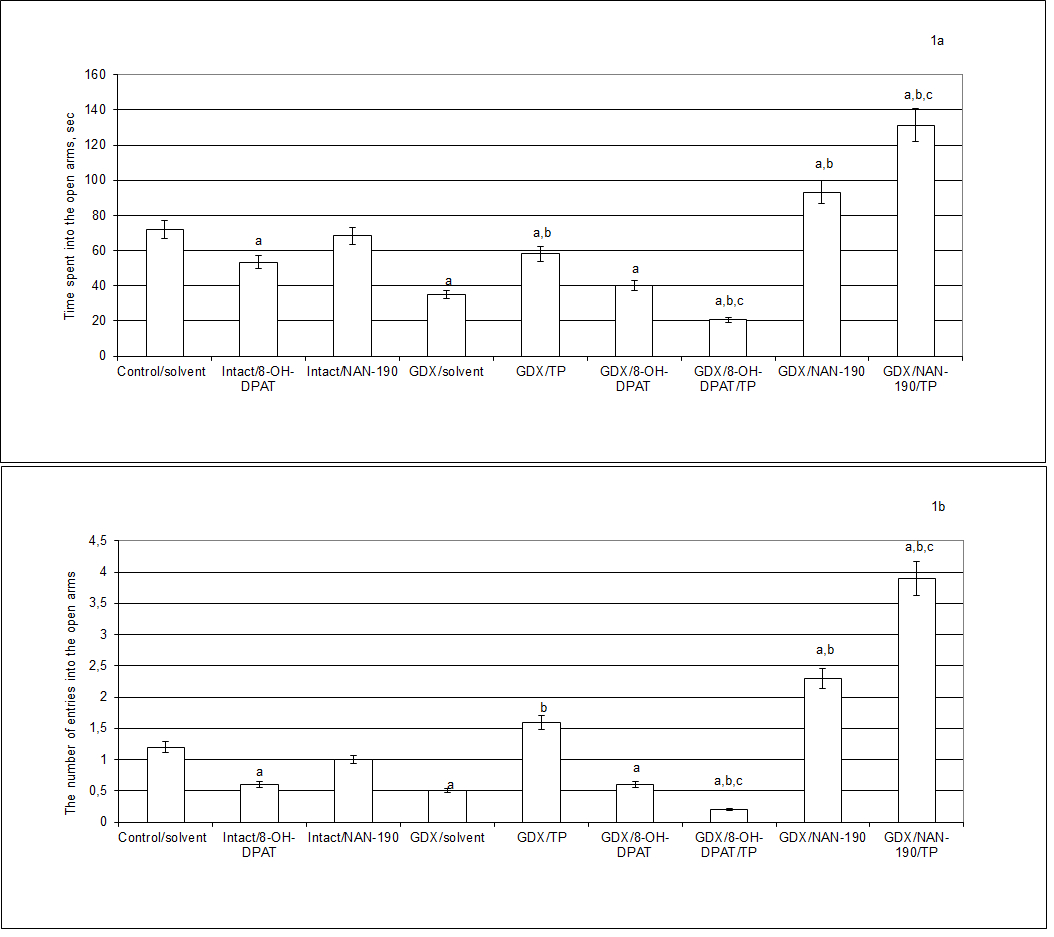
A two-way ANOVA revealed significant differences in the time spent into the open arms between hormone conditions ([F(5,32) = 7.4, P<0.0004]), between drug treatments [F(5,32) = 11.04, P<0.0003]), and an interaction between hormone condition and treatments ([F(5,32) = 1.84, P<0.001]) in the GDX rats. The post-hoc test revealed differences among the groups for anxiety-like behavior in the EPM (P<0.05). The intact/8-OH-DPAT rats demonstrated a significant decrease of the time spent into the open arms as compared to the control intact rats (p<0.05) (Fig. 1a).
The intact/NAN-190 rats failed to modify the time spent into the open arms as compared to the control group. Gonadectomy in male rats (GDX/solvent rats) resulted in a significant decrease of time spent into the open arms as compared to the control/solvent rats (p<0.05) (Fig. 1). TP supplementation (0.5 mg/kg, s.c.) caused an increase of time spent into the open arms in the GDX rats as compared to the GDX/solvent rats (p<0.05) (Fig. 1a). Although, the value of this parameter in the GDX/TP rats was higher than that of the GDX/solvent, it did not reach the value of control intact rats. Administration of 5-HT1A receptor agonist, 8-OH-DPAT (0,05 mg/kg, s.c.), significantly decreased time spent into the open arms in the GDX rats as compared to the intact/solvent (p<0.05) (Fig. 1a). Moreover, the time spent into the open arms values of the GDX rats given with combination of 8-OH-DPAT and TP were profoundly lower than that of the GDX/TP, GDX/solvent and control/solvent rats. Administration of 5-HT1A receptor antagonist, NAN-190 (0.1 mg/kg, i.p.), significantly increased time spent into the open arms in the GDX rats as compared to the intact/ solvent and GDX/solvent rats (p<0.05) (Fig. 1a). NAN-190 in combination with TP more significantly increased time spent into the open arms in the GDX rats as compared to the intact, GDX or GDX rats with oil solvent or TP alone (p<0.05) (Fig. 1a). The time spent into the open arms values of the GDX rats given with combination of NAN-190 and TP were profoundly higher than that of the GDX/solvent or GDX/TP rats and intact/solvent.
Similarly, significant differences in the number of entrances into the open arms were found between hormone conditions ([F(5,32) = 2.96, P<0.01]), between drug treatments [F(5,32) = 7.20, P<0.003]), and an interaction between hormone condition and treatments ([F(4,30) = 11.22, P<0.0001]) in the GDX males. The post-hoc test revealed differences among the groups for this parameter in the EPM (P<0.05). GDX/solvent rats displayed a significant decrease of the number of entries into the open arms as compared to the control/solvent rats (p<0.05) (Table 1). 8-OH-DPAT s.c. injection to intact male rats induced a decreased number of entries into the open arms as compared to the control rats (p<0.05) (Fig. 1b). The intact/NAN-190 rats did not change the number of entries into the open arms as compared to the control group. GDX/solvent rats displayed a significant decrease of the number of entries into the open arms as compared to the control/solvent rats (p<0.05) (Fig. 1b). Administration of TP to the GDX rats increased the number of entries into the open arms as compared to the GDX/solvent rats (p<0.05) (Fig. 1b). The GDX/8-OH-DPAT rats showed a significant decrease of the number of entries into the open arms as compared to the intact/solvent (p<0.05) (Fig. 1b). The number of entries into the open arms values of the GDX rats given with combination of 8-OH-DPAT and TP were profoundly lower than that of the GDX/TP, GDX/solvent and control/solvent rats. GDX/NAN-190 rats showed a significant increase of the number of entries into the open arms as compared to the intact/solvent and GDX/solvent rats (p<0.05) (Fig. 1b). The GDX/ NAN-190/TP rats also demonstrated an increase in the number of entries into the open arms as compared to the intact/solvent, GDX/solvent and GDX/TP rats (p<0.05) (Fig. 1b).
Figure 1. Effects of 8-OH-DPAT or NAN-190 chronic treatment on anxiety-like behavior of gonadectomized male rats in the elevated plus maze. a - P < 0.05 as compared to the control group of intact sham-operated rats, b - P < 0.05 as compared to the gonadectomized (GDX) male rats treated with oil solvent, c - P < 0.05 as compared to the gonadectomized (GDX) male rats treated with testosterone propionate (TP). Two weeks after gonadectomy, GDX rats were subjected by treatments with the solvent, TP (0.5 mg/kg, s.c.), 8-OH-DPAT (0.05 mg/kg, s.c.), NAN-190 (0.1 mg/kg, i.p.), 8-OH-DPAT in a combination with TP or NAN-190 in a combination with TP during 14 days. Each group comprised a minimum of 10 rats. All values were expressed as mean ± S.E.M. Comparisons between values were performed using two-way ANOVA test with between-subject factors of hormone conditions (GDX or GDX plus TP) and drug treatment (8-OH-DPAT or NAN-190) followed by Dunnett’s test for multiple comparisons post-hoc test.
Effects of 8-OH-DPAT and NAN-190 on the behavior of GDX males and GDX males treated with testosterone propionate in the open field test
The two-way ANOVA revealed significant differences in the crossing, rearing and grooming between hormone conditions ([F(5,32) = 5.44, P<0.05], [F(5,32) = 9.40, P<0.01], [F(5,32) = 19.34, P<0.01], respectively), between drug treatments [F(5,32) = 15.4, P<0.001], [F(5,32) = 11.56, P<0.05], [F(5,32) = 7.86, P<0.05], respectively), and an interaction between hormone condition and treatments ([F(5,32) = 3.8, P<0.01], [F(5,32) = 5.46, P<0.05], [F(5,32) = 4.02, P<0.05], respectively). The post-hoc test failed to reveal any alterations of behavioral values in the intact/8-OH-DPAT, intact/NAN-190, GDX/solvent and GDX/TP rats compared to the intact/solvent (Table 2, non-significant).
Neither GDX/8-OH-DPAT nor GDX/8-OH-DPAT/TP rats showed frequency of rearing and grooming behavior in GDX rats as compared to the GDX/solvent and GDX/TP rats (Table 2, non-significant). However, the post-hoc test revealed that GDX/8-OH/DPAT or GDX/8-OH-DPAT/TP rats demonstrated a significant increase in crossing behavior to the GDX/solvent and GDX/TP rats (p<0.05) (Table 2). NAN-190 or GDX/NAN-190/TP rats showed a significantly elevated grooming as compared to the GDX/TP, GDX/solvent and intact/solvent rats (p<0.05) (Table 2).
Discussion
In the present work, the effects of chronic 8-OH-DPAT or NAN-190 treatments for 14 days on anxiety-like behavior in male rats with androgen deficiency and TP supplementation were investigated. Endogenous androgens were removed by gonadectomy and the results of behavioral testing for the anxiety-related effects of 8-OH-DPAT or NAN-190 applications were compared in both GDX rats and GDX male rats treated with TP. For this purpose, the elevated plus maze (EPM) and open field test (OFT) were performed in this study.
The results of the present study showed that in GDX/solvent rats, there were marked anxiety-like behavior as assessed by EPM. The EPM is recognized as a valuable model able to predict anxiolytic- or anxiogenic-like effects of drugs in rodents (Blainski et al., 2010). It is designed to present the animal with a conflict between its natural tendency to explore a novel environment and its reluctance to move away from the sheltering walls and into the open environment in which the risk of falling or exposure to predators is much higher (Pellow et al., 1985). Although TP supplementation resulted in significant anxiolytic-like effect of GDX rats, the TP administration was not able to completely reduce anxiety-like behavior to the level of control intact animals. According to these results, we conclude that GDX rats display significant anxiety-related status while TP administration to the GDX rats attenuates the gonadectomy-induced anxiety-like behavior to some extent. This confirms the results of preclinical and clinical studies showing that testosterone administration can have anxiolytic-like effect in males (Young and Korszun, 2002; McDermott et al., 2012; McHenry et al., 2014).
8-OH-DPAT treatment failed to influence anxiety-like behavior in the GDX rats. Administration of 8-OH-DPAT in combination with TP in the GDX rats inhibited the anxiolytic-like effect of TP. 8-OH-DPAT application in the GDX rats, unlikely treatment with TP, significantly increased locomotor activity in the OFT and produced the anxious-like state in the EPM. However, the results from GDX/TP and the GDX/8-OH-DPAT/TP rats indicate that 8-OH-DPAT affects anxiety-related processes rather than motor function. The OFT results in this study suggested that 8-OH-DPAT administered alone or together with TP to the GDX rats resulted in the increased motor behavior.
NAN-190 treatment has a significant anxiolytic-like effect and completely able to remove anxiety-like profile induced by androgen deficiency in the GDX rats. Interestingly, administration of NAN-190 in combination with TP in the GDX rats potentiated the anxiolytic-like effects of both preparations. Taken together these results, it can be assumed that NAN-190 may behaves itself like androgenic-like substance. Moreover, NAN-190, unlikely treatment with TP, significantly increased grooming events in the OFT and so significantly reversed the anxiety-like profile in the GDX rats in the EPM. The results from GDX/TP rats and from the GDX/NAN-190/TP rats indicate that NAN-190 affects anxiety-related processes rather than behavioral reactions. Also, the results of the present study indicated that administration of NAN-190 in combination with TP could reverse the effect of the experimental model of androgen deficiency on anxiety-related profile. In the present study, only one concentration of TP was used because this concentration of TP has been shown anxiolytic-like effects in GDX rats (Fedotova, 2014, 2015). Different dosages and duration of NAN-190 or TP treatments should be tested in the future study.
Interestingly, the effects of chronic 8-OH-DPAT or NAN-190 treatments on anxiety-like behavior in the intact and GDX rats are completely opposite the results of the previous study which were obtained both intact and ovariectomized (OVX) female rats (Fedotova et al., 2004). Given together these data, it can be conclude that different effects of 8-OH-DPAT and NAN-190 on anxiety-like behavior in the GDX or GDX/TP male rats might suggest the different mechanisms of interaction between gonadal hormones and 5-HT1A receptor in the pathophysiology of anxiety.
There are some explanations for the anxiolytic-like effects of NAN-190 in the GDX/solvent and GDX/TP rats obtained in our present study using EPM paradigm. One possible explanation for the different effects of 8-OH-DPAT and NAN-190 is that repeated injections may have led to a delayed desensitization of somatodendritic 5-HT autoreceptors. Alternative mechanisms for the different effects of 8-OH-DPAT and NAN-190 is functional sensitization of hippocampal 5-HT receptors or increased postsynaptic responsiveness.
On the other hand, a dysregulation of the hypothalamo-pituitary-adrenal (HPA) axis is one of the most commonly described alterations that correlate with symptoms of mood disorders and other neurospsychiatric diseases (49,56). On the other hand, gonadal steroids play a critical role in brain development and can modulate HPA axis activity (Watson et al., 2002; Young and Korszun, 2002; Bingham et al., 2011). Effects of the gonadal hormones on HPA function have been demonstrated at different levels of the axis. In general, androgens inhibit HPA activity. Androgens inhibit corticotrophin releasing hormone expression (Bingham et al., 1994), and gonadectomy of adult male rats increases both adrenocorticotropin and corticosterone responses to physical and psychological stressors (Handa et al., 1994), an effect reversed by replacement with testosterone or the non-aromatizable androgen, 5β-dihydrotestosteone (Handa et al., 1994; Viau et al., 2003). Gonadectomized (GDX) rats also show greater stress-induced c-Fos expression and higher arginine vasopressin hnRNA levels than intact males, both of which are negatively correlated with plasma testosterone levels (Viau, 2002; Viau et al., 2003). The gonadal and stress hormone systems have been shown to interact with each other, resulting in reciprocal modulation of each other’s expression and function (Swaab et al., 2005). These interactions are likely to be particularly important for development of numerous neuropsychiatric disorders. These interactions are likely to be particularly important for development of numerous neuropsychiatric disorders.
Furthermore, studies have demonstrated interactions between 5-HT system and the hypothalamic–pituitary–adrenal axis (Owen et al., 1990; Ruggiero et al., 1999; Semont et al., 1999), traditionally regarded as central in the stress response of mammals (Moberg, 1985). 5-HT has a stimulatory effect on the HPA axis in humans and rodents that is mediated by the 5-HT1A receptor, only male rodents respond to 5-HT1A antagonism to show increased corticosterone responses to stress. Finally, the actions of gonadal hormones to mediate adaptive neuroendocrine and behavioral responses may be completely impaired in the face of chronic stress exposure (Van De Kar, 1991; Sheehan and Sheehan, 2007).
One of the explanation could be that NAN-190 modulates the activity of the HPG system and normalizes the impaired feedback interaction within the HPG axis of GDX rats. Our future investigations will aim to clarify how chronic NAN-190 treatment alters ACTH and LH levels in the blood and the brain after injection in male rats with an imbalance of androgens. Interactions between genomic and non-genomic effects of NAN-190 and TP cannot be excluded. It is possible that the time- and concentration-dependent involvement of the non-genomic and nuclear receptor mediated effects of testosterone might underlie the complex associations between testosterone and NAN-190. Taken together these data in can be assumed that NAN-190 modulate activity of HPA and HPG systems and normalize the impaired feed back interaction between the HPA and HPG axes.
Another possible explanation could be that NAN-190 could affect GABA terminals in the hippocampus. 5-HT1A receptors may also be located on glutamatergic terminals (Albert et al., 2014). Serotonin and GABA show a reciprocal modulatory function in the hippocampus (Sheline et al., 2003). This region is rich in both 5-HT and GABA terminals and receptors (Stanzione et al., 1984; Bitran et al., 1993). Projections from serotonergic neurons of the raphe nuclei terminate on GABAergic hippocampal interneurons increasing or decreasing their activity via serotonergic receptors (Halasy et al., 1992; Pick et al., 1995).
In our study, the possible NAN-190 effects in hippocampus may be useful to study the respective contribution direct of serotonin and/or indirect of GABA in the treatment of psychiatric diseases. Moreover, it has been proposed that anxiogenic-like action of 8-OH-DPAT might also be mediated through activation of 5-HT7 receptors (Hedlund et al., 2004; Mombereau et al., 2010). Further studies are necessary to determine if modulation of 5-HT7 receptors contribute to the effects of 8-OH-DPAT.
Thus, the present data of the study indicates that chronic NAN-190 treatment on anxiety-related behavior after gonadectomy can be explained by its direct and/or indirect dual action on emotional functions of the brain and modifications of HPG or/and HPA axes. Taken together, it can be proposed that the positive effect of chronic NAN-190 treatment on anxiety-related brain function after gonadectomy is connected with its mutual and complex action on the numerous neurochemical and neurohormonal pathways. Other kind of studies is obviously necessary to elucidate the detail mechanism of action of NAN-190 in the brain.
Conclusion
Thus, the results of this study suggest that chronic 8-OH-DPAT and NAN-190 treatments can modify anxiety-like profile in opposite direction in GDX and GDX/TP male rats. The data also indicate that the combination of NAN-190 and TP is more effective than TP alone in GDX rats inducing a more profound anxiolytic-like effect in the EPM. Furthermore, this is the first study to show a beneficial effect of chronic NAN-190 administration on anxiety-related state in male rats with low androgen levels. This work promotes more effective creating of the novel therapeutic targets and strategies for anxiety treatment in subjects with androgen imbalance.
There are no any potential conflict interests for this article.
| Attachment | Size |
|---|---|
| 400.38 KB |
ALBERT P.R. & LE FRANCOIS B. (2010). Modifying 5-HT1A receptor gene expression as a new target for antidepressant therapy. Front Neurosci, 4, 1-7.
ALBERT P.R., VAHID-ANSARI F. & LUCKHART C. (2014). Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Front Behav Neurosci, 8, 1-13.
ALTEMUS M. (2006). Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav 50, 534-544.
BANDELOW B., ZOHAR J., HOLLANDER E., KASPER S.M. & MOLLER H.-J. (2008). WFSBP Task force on treatment guidelines for anxiety obsessive-compulsive post-traumatic stress disorders, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders – first revision.World J Biol Psy, 9, 248-312.
BARNES N.M. & SHARP T. (1999). A review of central 5-HT receptors and their function. Neuropharmacol, 38, 1083-1152.
BINGAMAN E.W., MAGNUSON D.J., GRAY T.S. & HANDA R.J. (1994). Androgen inhibits the increase in hypothalamic cortictropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinol, 59, 228-234.
BINGHAM B., GRAY M., SUN T., VIAU V. (2011). Postnatal blockade of androgen receptors or aromatase impair the expression of stress hypothalamic-pituitary-adrenal axis habituation in adult male rats. Psychoneuroendocrinol. 36, 249-257 (2011).
BITRAN D., KELLOGG C.K. & HILVERS R.J. (1993). Treatment with an anabolic-androgenic steroid affects anxiety-related behavior and alters the sensitivity of cortical GABAA receptors in the rat. Horm Behav 27, 568-583.
BLAINSKI A., PICCOLO V.K., MELLO J.C.P. & DE OLIVEIRA R.M.W. (2010). Dual effects of crude extracts obtained from Petiveria alliacea L. (Phytolaccaceae) on experimental anxiety in mice. J Ethnopharmacol, 128, 541-544 (2010).
BLIER P. & WARD N.M. (2003). Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psych, 53, 193-203.
CELADA P., BORTOLOZZ I.A. & ARTIGAS F. (2013). Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs, 27, 703-716.
DAVIDSON R.J. (2000). Anxiety, depression and emotion. New York, NY: Oxford University Press.
DE VRY J. (1995). 5-HT1A receptor agonists: recent developments and controversial issues. Psychopharmacol (Berl), 121, 1-26.
EDINGER K.L. & FRYE C.A. (2005). Testosterone’s anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinol, 30, 418-430.
EDINGER K.L. & FRYE C.A. (2006). Intrahippocampal administration of an androgen receptor antagonist, flutamide, can increase anxiety-like behavior in intact and DHT-replaced male rats. Horm Behav, 50, 216-222.
FEDOTOVA J., HARTMANN G., LENARD G. & SAPRONOV N. (2004). Effects of 5-HT1A receptor agonist and antagonist on anxiety in intact and ovariectomized female rats. Acta Physiol Hung, 91, 175-184.
FEDOTOVA J. (2014). Effects of stimualtion and blockade of D2-dopaminergic receptors on behavior in gonadectomized male rats. Ross Fiziol Zh Im IM Sechenova, 100, 1374-1381.
FEDOTOVA J. (2015). Blockade of D2-like dopamiergic receptor corrects anxiety-like behaviour in gonadectomized male rats treated with low dose of testosterone. Eur Psych, 30, Suppl. 1, 541.
FERNANDEZ-GUASTI A. & MARTINEZ-MOTA L. (2003). Orchidectomy sensitizes male rats to the action of diazepam on burying behavior latency: role of testosterone. Pharmacol Biochem Behav, 75, 473-479.
FERNANDEZ-GUASTI A. & MARTINEZ-MOTA L. (2005). Anxiolytic-like actions of testosterone in the burying behavior test: role of androgen and GABA benzodiazepine receptors. Psychoneuroendocrinol, 30, 762-770.
FRYE C.A. & SELIGA A.M. (2001). Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci, 1, 371-381.
FRYE C.A. & EDINGER K.L. (2004). Testosterone's metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav, 78, 473-481.
FRYE C.A., EDINGER K & SUMIDA K. (2008). Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacol, 33, 1049-1061.
GARCIA-GARCIAA A., NEWMAN-TANCREDIB A. & LEONARDOA E.D. (2014). 5-HT1A receptors in mood and anxiety: recent insights into autoreceptor versus heteroreceptor function. Psychopharmacol (Berl), 231, 623-636.
GLITZ D.A. & POHL R. (1991). 5-HT partial agonists. What is their 1A future? Drugs, 41, 11-18.
HALASY K., MIETTINEN R., SZABAT E. & FREUND T.F. (1992). GABAergic interneurons are the major postsynaptic targets of median raphe afferents in the rat dentate gyrus. Eur J Neurosci, 4, 144-153.
HANDA R.J., BURGESS L.H., KERR J.E., O’KEEFE J.A. (1994). Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav, 28, 464-476 (1994).
HARTO N.E., BRANCONNIER R.J., SPERA K.F. & DESSAIN E.C. (1988). Clinical profile of gepirone, a nonbenzodiazepine anxiolytic, Psychopharmacol Bull, 24, 154-160.
HEDLUND P.B., KELLY L., MAZUR C., LOVENBERG T., SUTCLIFFE J.G. & BONAVENTURE P. (2004). 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol, 487, 125-132.
HODOSY J., ZELMANOVA D., MAJZUNOVA M., FILOVA B., MALINOVA M., OSTATNIKOVA D. & CELEC P. (2012). The anxiolytic effect of testosterone in the rat is mediated via the androgen receptor. Pharmacol Biochem Behav, 102, 191-195.
HOGERVORST E. (2013). Effects of gonadal hormones on cognitive behaviour in elderly men and women. J Neuroendocrinol, 25, 1182-1195.
KESSLER R.C., ANGERMEYER M., ANTHONY J.C. & R D.G. (2007). Lifetime prevalence and age of onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychi, 6, 168-176.
KHAKPAI F. (2014). The effect of opiodergic system and testosterone on anxiety behavior in gonadectomized rats. Behav Brain Res, 125, 70-77.
KRONKE K., SPITZER R.L. & WILLIAMS J.B. (2007). Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med, 146, 317-325.
LUCKI I., SINGH A. & KREISS D.S. (1994). Antidepressant-like behavioral effects of serotonin receptor agonists. Neurosci Biobehav Rev, 18, 85-95.
MARSHALL K.M. (2011). Introduction to the interaction between gonadal steroids and the central nervous system. Curr Top Behav Neurosci, 8, 1-13.
MARTIN-MERINO E., RUIGOMEZ A., WALLANDER M.A., JOHANSSON S. & GARSIA-RODRIGUES L.A. (2010). Prevalence, incidence, morbidity and treatment patterns in a cohort of patients diagnosed with anxiety in UK primary care. Fam Pract, 27, 9-16.
MARTINEZ-CONDE E., LERET M.L. & DIAZ S. (1985). The influence of testosterone in the brain of the male rat on levels of serotonin (5-HT) and hydroxyindole-acetic acid (5-HIAA). Comp Biochem Physiol, C 80, 411-414.
MATSUDAA T. (2013). Neuropharmacologic studies on the brain serotonin 1A receptor using the selective agonist osemozotan. Biol Pharm Bull 36, 1871-1882.
MCDERMOTT C.M., LIU D. & SCHRADER L.A. (2012). Role of gonadal hormones in anxiety and fear memory formation and inhibition in male mice. Physiol Behav, 105, 1168-1174.
MCHENRY J., CARRIER N., HULL E. & KABBAJ M. (2014). Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol, 35, 42-57.
MENZAGHI F., HOWARD R.L., HEINRICHS S.C., VALE W., RIVIER J. & KOOB G.F. (1994). Characterization of a novel and potent corticotropin-releasing factor antagonist in rats. J. Pharmacol Exp Ther, 269, 564-572.
MOBERG G.P. (1985). Biological responses to stress: key to assessment of animal well-being? In: Moberg GP, editor. Animal stress, USA: American Physical Society, 27-49.
MOMBEREAU C., GUR T.L., ONKSEN J. & BLENDY J.A. (2010). Differential effects of acute and repeated citalopram in mouse models of anxiety and depression. Int. J. Neuropsychopharmacol, 13, 321-334.
OWEN M.J, EDWARDS E. & NEMEROFF C.B. (1990). Effects of 5-HT1A receptor agonists on hypothalamo-pituitary–adrenal axis activity and corticotropin-releasing factor containing neurons in the rat brain. Eur J Pharmacol, 190, 113-122 (1990).
PAZOS A. & PALACIOS J.M. (1985). Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res, 346, 205-230.
PELLOW S., CHOPIN P., FILE S.E. & BRILEY M. (1985). Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods, 14, 149-167.
PICK U., HARAMAKI N., CONSTANTINESCU A., HANDELMAN G.J., TRITSCHLER H.J. & PACKER L. (1995). Glutathione reductase and lipoamide dehydrogenase have opposite stereo specificities for alpha-lipoic acid enantiomers. Biochem Biophys Res Commun, 206, 724-730.
POPE H.G. JR, COHANE G.H., KANAYAMA G., SIEGEL A.J. & HUDSON J.I. (2003). Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psych, 160, 105-111.
RUGGIERO D.A., UNDERWOOD M.D., RICE P.M., MANN J.J. & ARANGO V. (1999). Corticotropic-releasing hormone and serotonin interact in the human brainstem: behavioral implications. Neurosci, 91, 1343-1354.
SEMONT A. FACHE M.-P., OUAFIK L.’H., HERY M., FAUDON M. & HERY F. (1999) Effects of serotonin inhibition on glucocorticoid and mineralocorticoid expression in various brain structures. Neuroendocrinol, 69, 121-129.
SHEEHAN D.V. & SHEEHAN K.H. (2007). Current approaches to the pharmacologic treatment of anxiety disorders. Psychopharmacol Bull, 40, 98-109.
SHELINE Y.I., GADO M.H. & KRAEMER Y.C. (2003). Untreated depression and hippocampal volume loss. Am J Psych, 160, 1516-1518.
SOMERS J.M., GOLDNER E.M., WARAICH P. & Hsu L. (2006). Prevalence and incidence studies of anxiety disorders: a systematic review of the literature. Can J Psych, 51, 100-113.
STANZIONE P., CALABRESI P., MERCURI N. & BEMARDI G. (1984) Dopamine modulates CA1 hippocampal neurones by elevating the threshold for spike generation: an in vitro study. Neurosci, 13, 1105-1116.
SWAAB D.F., AI-MIN B. & LUCASSEN P.J. (2005). The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev, 4, 141-194.
VAN DE KAR L. (1991) Neuroendocrine pharmacology of serotonergic (5-HT) neurons. Ann Rev Pharmacol Toxicol, 31, 289-320.
VERGÉ D., DAVAL G., MARCINKIEWICZ M., PATEY A., EL MESTIKAWY S. & GOZLAN H. (1986) Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J Neurosci, 6, 3474-3482.
VIAU V. (2002) Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol, 14, 506-513.
VIAU V., LEE P., SAMPSON J. & WU J. (2003) A testicular influence on restraint-induced activation of medial parvocellular neurons in the paraventricular nucleus in the male rat. Endocrinol, 144, 3067-3075.
WATSON S., GALLAGHER P., DEL-ESTAL D., HEARN A., FERRIER I.N. & YOUNG A.H. (2002) Hypothalamic-pituitary- adrenal axis function in patients with chronic depression. Psychol Med, 32, 1021-1028.
WITTCHEN H.U. (2002) Generalized anxiety disorder: prevalence, burden, and cost to society. Depress Anxiety, 16, 162-171.
XU L., ANWYL R., De VRY J. & ROWAN M.J. (1997). Effect of repeated ipsapirone treatment on hippocampal excitatory synaptic transmission in the freely behaving rat: role of 5-HT receptors 1A and relationship to anxiolytic effect. Eur J Pharmacol, 323, 59-68.
YOUNG E.A. & KORSZUN A. (2002). The hypothalamic-pituitary-gonadal axis in mood disorders. Endocrinol Metab Clin North Am, 31, 63-78.
ZHANG L., MA W., BARKER J.L. & RUBINOW D.R. (1999). Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neurosci, 94, 251-259.