Nitric oxide (NO) signalling contributes to many biological processes involved in activity-dependent fine tuning of neuronal communication. NO is involved in early developmental signalling of the nervous system and is associated with pathological pathways and age-related decline in neuronal function, thus playing a critical role in regulating neuronal function in physiology and disease. Here we assessed the effects of modulating endogenous neuronal nitric oxide synthase (NOS) activity on synaptic function, specifically on neurotransmitter release at the glutamatergic Drosophila neuromuscular junction (NMJ). We found that the absence of NOS activity enhanced synaptic release at the NMJ and conversely, overexpression of NOS diminished transmitter release. The effects of alterations in NO signalling are the consequence of acute signalling at the synapse as we did not observe any developmental changes in NMJ morphology or synaptic parameters, such as expression of the active zone protein, bruchpilot, which could account for changes in release. Ultrastructural analysis did not show any developmental effects in boutons from larvae with reduced NOS activity. Together, our data present evidence for a negative regulation of transmitter release by NO which has implications for physiological synaptic function but also pathological and age-related dysregulation of synaptic signalling.
Introduction
Nitric oxide (NO) is involved in a broad variety of signalling pathways contributing to neuronal plasticity, immune responses, vascular signalling, development and survival. It was originally identified as endothelium-derived relaxing factor (EDRF) mediating relaxation of blood vessels (Furchgott & Zawadzki, 1980). In mammals, NO is synthesized by nitric oxide synthases (NOS) through the conversion of L-arginine to NO and L-citrulline (Knowles & Moncada, 1994). Three isoforms of NOS have been identified in the central nervous system (CNS): neuronal NOS (nNOS), inducible NOS (iNOS) and endothelial NOS (eNOS) (Alderton et al., 2001), with the activation of the soluble guanylyl cyclase (sGC) and cGMP production being the canonical pathway and a primary target of NO actions (Garthwaite et al., 1988). Down-stream transduction of NO signalling can be via cGMP regulated cyclic nucleotide-gated ion channels, activation of protein kinase G and protein phosphorylation or direct actions on proteins via post-translational modifications (PTM, S-nitrosylation and 3-nitrotyrosination) (Hardingham et al., 2013; Garthwaite, 2016). These NO-mediated PTMs in particular have become increasingly recognised as regulators of specific target proteins (Knott & Bossy-Wetzel, 2009). S-nitrosylation is a non-enzymatic and reversible PTM resulting in a covalent addition of an NO group to a cysteine (Cys) thiol/sulfhydryl leading to the generation of S-Nitrosothiols (S-NO), and this pathway participates in a huge number of physiological events including cellular trafficking (Ozawa et al., 2008), circulation (Singel & Stamler, 2005), apoptotic pathways and neuronal signalling (Cho et al., 2009). Throughout the nervous system NO modulates synaptic function by multiple mechanisms, including regulation of transmitter release and activity-dependent plasticity phenomena. NO regulates transmitter release (GABA, dopamine, noradrenaline) and, depending on the system and concentrations of NO studied, the effects range from strongly inhibiting to facilitating actions. As such NO can directly modulate SNAP-25 function to enhance release via post-translational modification signalling (Di Stasi et al., 2002) and nitrosylate syntaxin (Wiseman et al., 2011) which leads to reduced transmitter release and exacerbates the detrimental effects of NO on neurotransmission. NMDA receptors are negatively regulated by NO (Choi et al., 2000) leading to a nitrergic negative feedback regulation of excitotoxicity.
Mammalian nNOS exists in different splice variants, namely nNOSβ and nNOSγ, both of which lack the amino terminal PDZ domain (Brenman et al., 1996). Drosophila possesses endogenous NO signalling (Regulski & Tully, 1995; Wildemann & Bicker, 1999; Stasiv et al., 2001) and wild-type Drosophila NOS (dNOS), which has a 43% amino acid sequence identity compared to rat nNOS (Regulski & Tully, 1995) and produces NO in a Ca2+/calmodulin-dependent manner.
As NO has numerous critical functions, it would be expected that a lack of NOS activity would be lethal during developing. However, a role for NO in developmental survival has not yet been demonstrated. Although a genetic analysis of NOS function in vertebrates is complicated by the presence of three NOS genes and different splice variants, mice with a homozygous ablation of any single NOS gene are viable, animals with two NOS genes knocked out show drastically reduced viability and triple knockout animals have not yet been generated. The use of Drosophila to investigate NO dependent functions is advantageous due to the presence of only one NOS gene. As such, various forms of dominant negative dNOS have been generated to investigate developmental involvement of NO (Enikolopov et al., 1999; Stasiv et al., 2001; Stasiv et al., 2004) and it has been shown that NO has an anti-proliferative function during Drosophila development, controlling the balance between cell proliferation and cell differentiation (Kuzin et al., 1996). Other fly lines with strongly reduced dNOS activity have been generated (NOSC and NOS∆15, both of which show NOS activities comparable to NOS inhibited w1118) rendering them NOS ‘null’ (Regulski et al., 2004; Yakubovich et al., 2010).
In our study we investigated the effects of NO on synaptic release using genetic manipulations of NO signalling and found that enhanced nitrergic activity, induced by an endogenous increase of NOS activity compromises neurotransmitter release at the Drosophila NMJ and conversely, lack of NOS activity enhances release.
Methods
Fly stocks: Flies were raised on standard maize media at 25°C at a 12h LD cycle. w1118 larvae were used as controls (wild-type; WT). NOS∆15 / NOSC (Regulski et al., 2004) lines were kindly provided by Patrick O'Farrell (UCSF, San Francisco, California USA). NOSΔ15 deletion removes sequences encoding residues 1–757, encompassing the entire oxygenase domain and including regions that bind the catalytic heme and the substrate rendering the lines NOS ‘null’ (Yakubovich et al., 2010). The dNOS1 line with dNOS overexpression under the heat-shock promoter was kindly provided by Boris Kuzin (Koltsov Institute of Developmental Biology, Russia) (Kuzin et al., 1996; Stasiv et al., 2001).
Electrophysiology. TEVC recordings were performed as described previously (Robinson et al., 2014). Sharp-electrode recordings were made from ventral longitudinal muscle 6 in abdominal segments 2 and 3 of 3rd instar larvae using pClamp 10, an Axoclamp 900A amplifier and Digidata 1440A (Molecular Devices, USA) in hemolymph-like solution 3 (HL-3) (Stewart et al., 1994). Recording electrodes (20-50 MΩ) were filled with 3 M KCl. mEJCs were recorded in the presence of 0.5 μM tetrodotoxin (Tocris, UK). All synaptic responses were recorded from muscles with input resistances ≥4 MΩ and resting potentials more negative than −60 mV at 25°C as differences in recording temperature cause changes in glutamate receptor kinetics and amplitudes (Postlethwaite et al., 2007). Holding potentials were -60 mV. The extracellular HL-3 contained (in mM): 70 NaCl, 5 KCl, 20 MgCl2, 10 NaHCO3, 115 sucrose, 5 trehalose, 5 HEPES and 1.5 CaCl2. Average single eEJC amplitudes (stimulus: 0.1 ms, 1-5 V) are based on the mean peak eEJC amplitude in response to ten presynaptic stimuli (recorded at 0.2 Hz). Nerve stimulation was performed with an isolated stimulator (DS2A, Digitimer). All data were digitized at 10 kHz and for miniature recordings, 10 s recordings we analyzed to obtain mean mEJC amplitudes, decay and frequency (f) values. Quantal content (QC) was estimated for each recording by calculating the ratio of eEJC amplitude/average mEJC amplitude followed by averaging recordings across all NMJs for a given genotype. mEJC and eEJC recordings were off-line low-pass filtered at 500 Hz and 1 kHz, respectively. Materials were purchased from Sigma-Aldrich (UK) unless otherwise stated.
Cumulative postsynaptic current analysis. The apparent size of the vesicle pool was probed by the method of cumulative eEJC amplitudes (Schneggenburger et al., 1999). Muscles were clamped to −60 mV and eEJC amplitudes during a stimulus train (50 Hz, 500 ms) were calculated as the difference between peak and baseline before stimulus onset of a given eEJC. Receptor desensitisation was not blocked as it did not affect eEJC amplitudes since a comparison of the decay of the first and the last eEJC within a train did not reveal any significant difference in decay kinetics. The number of release-ready vesicles was obtained by back-extrapolating a line fit to the linear phase of the cumulative eEJC plot (the last 200 ms of the train) to time zero. The number of release-ready vesicles was then obtained by dividing the cumulative eEJC amplitude at time zero by the mean mEJC amplitude recorded in the same cell. To calculate the quantal content in the train, we used mean mEJC amplitudes measured before the train.
Immunohistochemistry (IHC). 3rd instar larvae were dissected in ice-cold PBS then fixed in 4% Paraformaldehyde. After permeabilisation with PBS-0.1% Triton (PBS-T) and blocking with PBS-T containing 0.2% BSA and 2% normal goat serum, larval fillets were incubated at 4°C overnight in solutions of primary antibody. The following antibody dilutions were used: NC82 (supernatant) anti-Brp (Bruchpilot; Developmental Studies Hybridoma Bank) 1:200 dilution. After 3x10min washes in PBS-T, larvae were incubated with AlexaFluor 488 goat anti-HRP (Jackson Immuno Research) and AlexaFluor 546 goat anti-mouse 1:500 dilution for 90 min at room temperature. Larvae were mounted using Vectashield mounting medium (Vector Labs) and NMJ 6/7 (segments A2 and A3) images were acquired with a Zeiss laser-scanning confocal microscope (LSM 510, Carl Zeiss). Image analysis was performed of z-stack images with ZEN (Carl Zeiss) and Volocity software and shown in figures as maximal projections.
Western blotting. Protein extracts from adult fly heads were separated by SDS-PAGE followed by immunoblotting with a rabbit dNOS antibody (kind gift from P.H. O’Farrell lab) at 1:400 dilution and α-tubulin at 1:1000 dilution.
Electron microscopy. Third instar larvae were ‘filleted’ in phospate-buffered saline at room temperature and then fixed in 2% (wt/vol) glutaraldehyde in 0.1M sodium cacodylate buffer (pH 7.4) at 4 oC overnight. They were post-fixed with 1% (wt/vol) osmium tetroxide/1% (wt/vol) potassium ferrocyanide for 1h at room temperature and then stained en bloc, overnight, with 5% (wt/vol) aqueous uranyl acetate at 4 oC , dehydrated and embedded in Taab epoxy resin (Taab Laboratories Equipment Ltd, Aldermaston, UK). Semi-thin sections, stained with toluidine blue, were used to identify areas containing synaptic regions (muscle 6/7 in regions A2/A3). Ultra-thin sections were cut from these areas, counterstained with lead citrate and examined in an FEI Talos transmission electron microscope (FEI Company (Thermo Fisher Scientific Inc.), Hillsboro, Oregon USA). Images were recorded using an FEI Ceta-16M CCD camera with 4k X 4k pixels. SV measurements were made using ImageJ software. A total of ~500-600 SVs were measured in 5-10 boutons from three animals per genotype.
Statistics. Statistical analysis was performed with Prism 6 and InStat 3 (Graphpad Software Inc., San Diego, USA). Statistical tests were carried out using an ANOVA test where applicable with a posteriori test (One-Way ANOVA with Tukey's multiple comparisons test) or unpaired Student’s t-test as indicated. Data are expressed as mean ± SEM where n is the number of boutons, NMJs or fly heads as indicated and significance is shown as *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001.
Results
Glutamatergic transmission is negatively regulated by NO
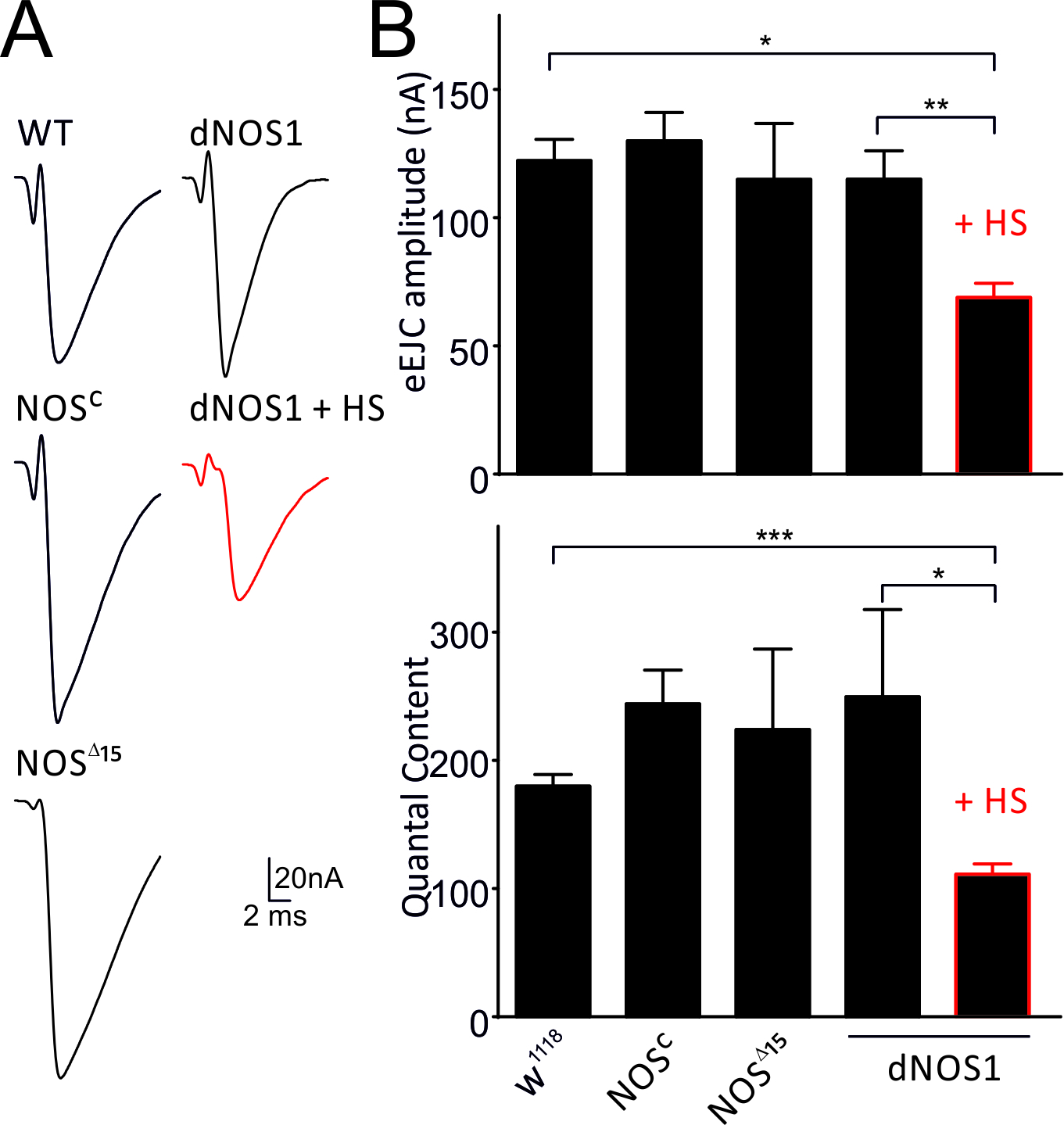
To examine the impact of NO signalling on glutamatergic transmission we characterised synaptic release at the NMJ of Drosophila 3rd instar larvae, which either overexpress dNOS or do not possess endogenous NOS activity and compared their responses to wild-type animals. As such we used dNOS1 overexpressing larvae (expression induced by heat-shock, HS) (Stasiv et al., 2001), NOSC / NOS∆15 (Yakubovich et al., 2010) and w1118 wild-type (WT) larvae. In all three genotypes we measured evoked excitatory synaptic currents (eEJC) and miniature EJCs (mEJC), and characterised the size of available vesicles within the readily releasable pool using the cumulative postsynaptic current analysis (Kuromi & Kidokoro, 2002). We found that eEJC amplitudes are reduced in larvae overexpressing dNOS1 for 24-48 hours compared to wild-type controls or non-heat shocked dNOS1 larvae (Fig. 1). Assuming that single quanta summate linearly in eEJCs and mEJCs and evoked responses arise from the same pool of vesicles at these low stimulus frequencies, the quantal content (QC) – the number of vesicles released per action potential was estimated as the ratio of the eEJC and mEJC amplitudes per NMJ. Analysis revealed that dNOS1 overexpression leads to a reduction of the QC indicating pre-synaptic actions of NO (Fig. 1).
Figure 1. Evoked release and quantal content are reduced following enhanced nitrergic activity. A, single eEJC recordings for genotypes and conditions indicated. B, eEJC amplitudes and quantal content are reduced following activation of NO signalling (dNOS+HS and NO application). Data denote Mean ± SEM, n=10-12 NMJs, ANOVA with post hoc Tukey-Kramer was used for comparisons, *p<0.05, **p<0.01, ***p<0.001.
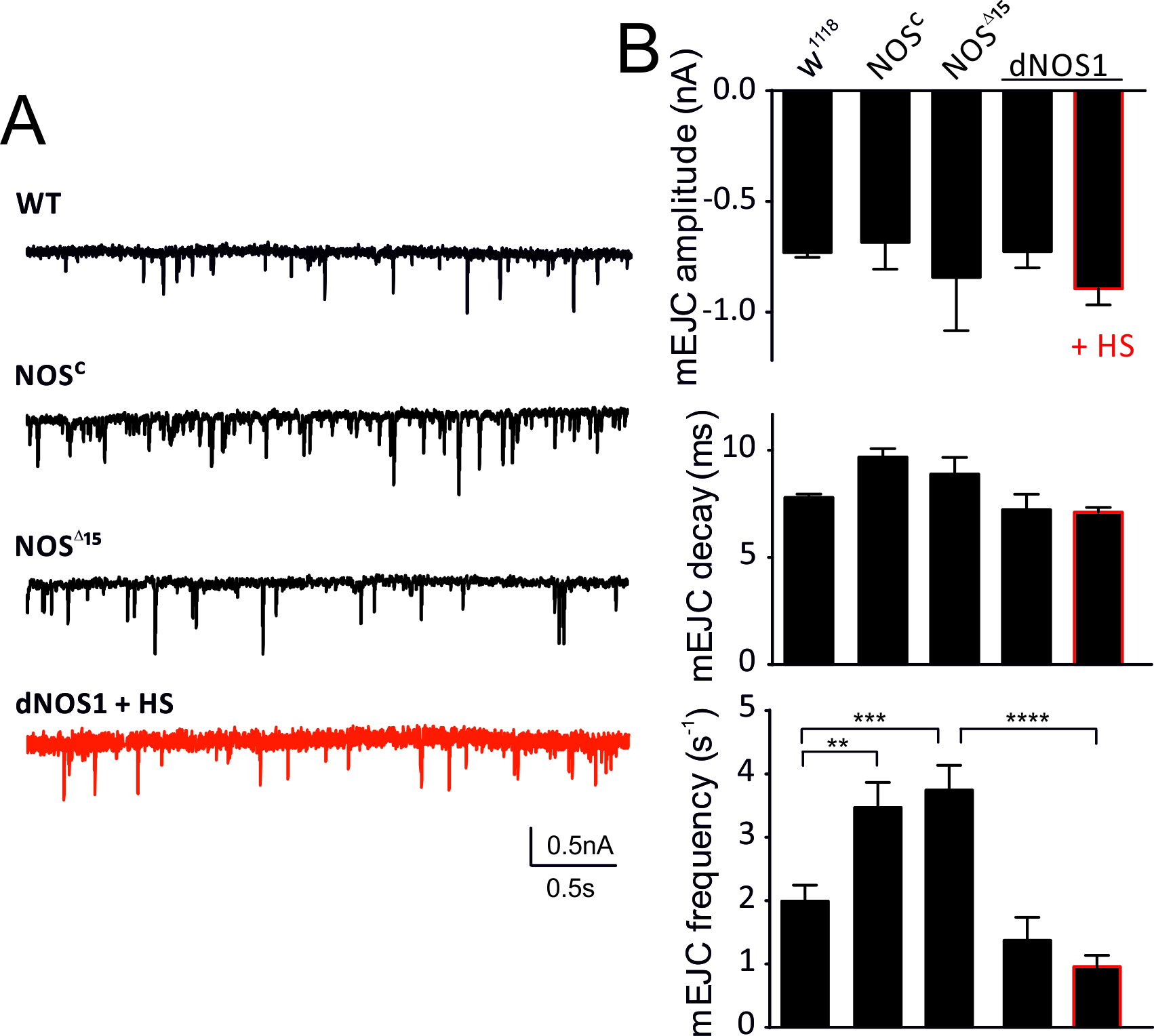
We further assessed spontaneous miniature responses in larvae with reduced or enhanced NOS expression and detected changes in release frequencies of mEJCs between NOSC / NOS∆15 and dNOS1 over-expressors but not in mEJC amplitudes or mEJC decay kinetics (Fig. 2). Reduction of NOS activity further enhanced spontaneous release frequency relative to w1118 WT suggesting that NO negatively influences presynaptic vesicle release.
Figure 2. NOS activity reduces spontaneous release frequency. A, mEJC recordings for genotypes and conditions indicated. B, mEJC parameters (top to bottom: amplitude, decay, frequency); amplitudes and decay are not affected by NO, the frequency is increased compared to WT in NOSC and NOS∆15 larvae. NOS overexpression (HS) reduces mEJC frequencies. Data denote Mean ± SEM, n=10-12 NMJs, ANOVA with post hoc Tukey-Kramer was used for comparisons, **p<0.01, ***p<0.001, ****p<0.0001.
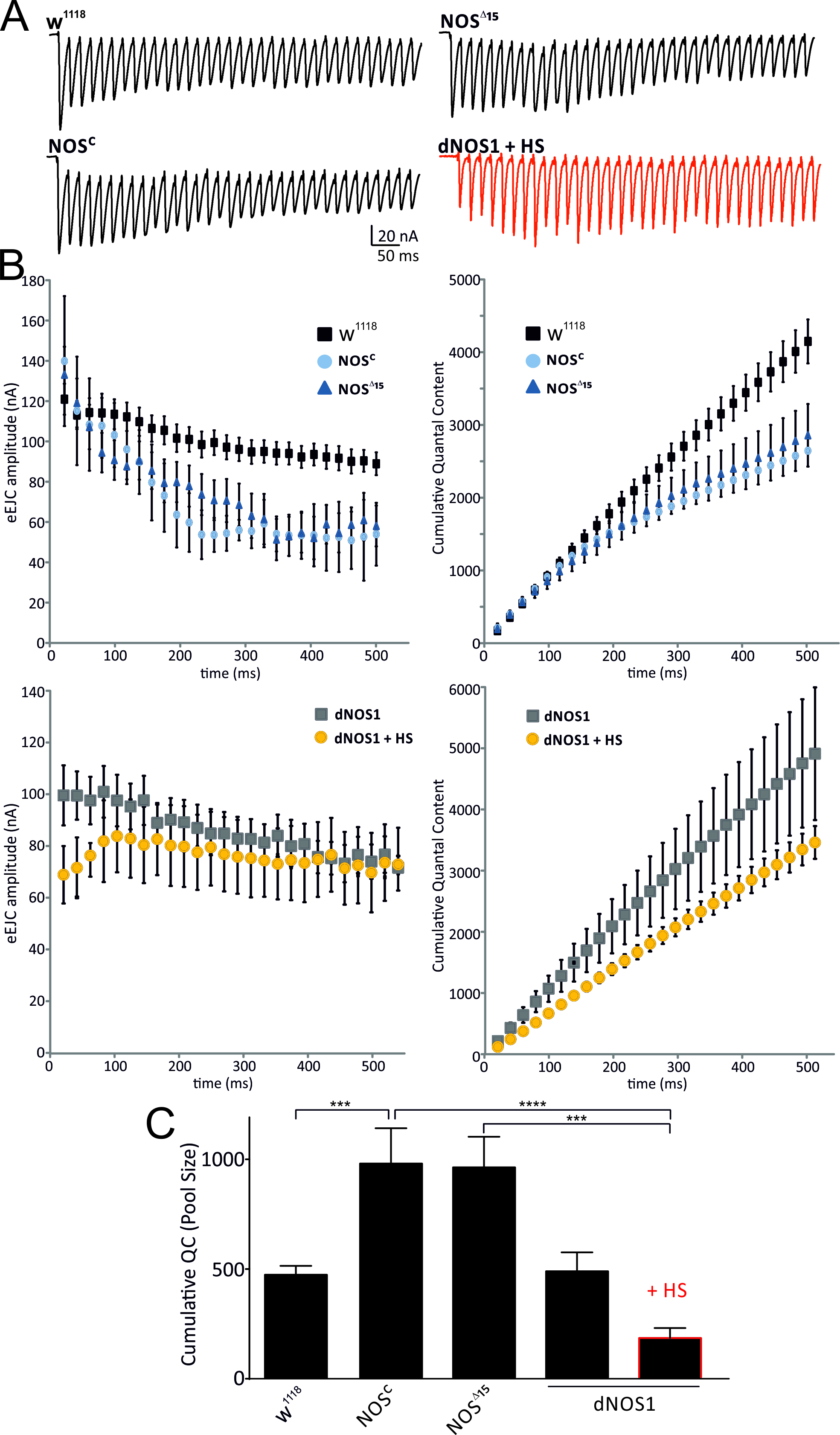
To assess whether NO can affect the availabilities of release-ready vesicles from presynaptic pools, we estimated the size of ready-releasable pools using cumulative postsynaptic current analysis (Kuromi & Kidokoro, 2002; Weyhersmuller et al., 2011). These estimations showed that the presence of NO reduces the number of releasable vesicles as dNOS1 expressing (+HS) and NOSC / NOS∆15 larvae resulted in opposite changes of pool sizes compared to w1118 WT larvae (Fig. 3).
Figure 3. The size of the ready releasable pool is reduced by nitrergic activity. A, eEJC recordings of a 50 Hz train, 500 ms, for genotypes and conditions indicated. B, left diagrams: mean eEJC amplitudes within the train. Right: cumulative quantal content with yielding an estimated readily releasable vesicle pool size (intercept with y-axis following back-extrapolation of the data points from the last 200 ms). C, mean cumulative quantal contents estimated by back-extrapolated linear regression to the last 200 ms to time 0 yielding an estimated reserved pool size (intercept with y-axis). Data denote Mean ± SEM in all graphs, n=10-12 NMJs, ANOVA with post hoc Tukey-Kramer was used for comparisons, ***p<0.001, ****p<0.0001.
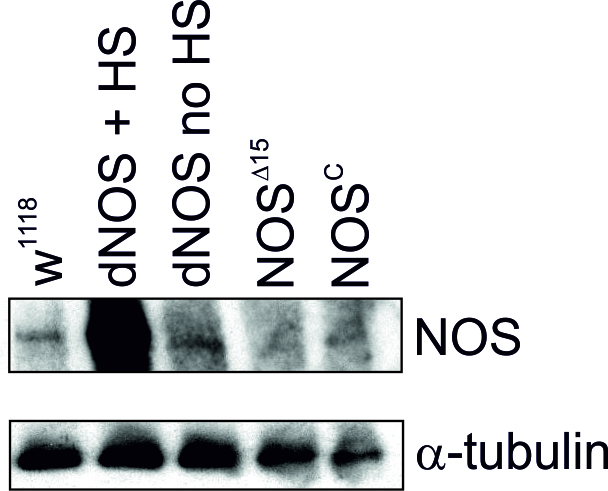
To confirm expression levels of NOS in the genotypes employed in our studies we performed Western blot analysis which showed lack of and strong levels of NOS expression in NOSC / NOS∆15 and dNOS1 (+HS) larvae, respectively relative to w1118 WT (Fig. 4). Together, these data suggest that activated NO signalling compromised vesicular release, as seen in reduced numbers of releasable vesicles per incoming action potential as well in the decreased frequency of spontaneously released vesicles under conditions of elevated nitrergic activity. Conversely, reduction of NOS expression leads to enhanced release, which supports the notion of inhibitory NO actions on release.
Figure 4. Altered NOS expression levels in larvae.Western blot analysis of different genotypes shows enhanced expression levels of dNOS following heat shock (HS) induction and no or reduced protein levels in NOS∆15 and NOSC fly heads, respectively relative to WT w1118. n=3 fly heads each.
Alterations in NOS activity do not cause developmental effects at the NMJ
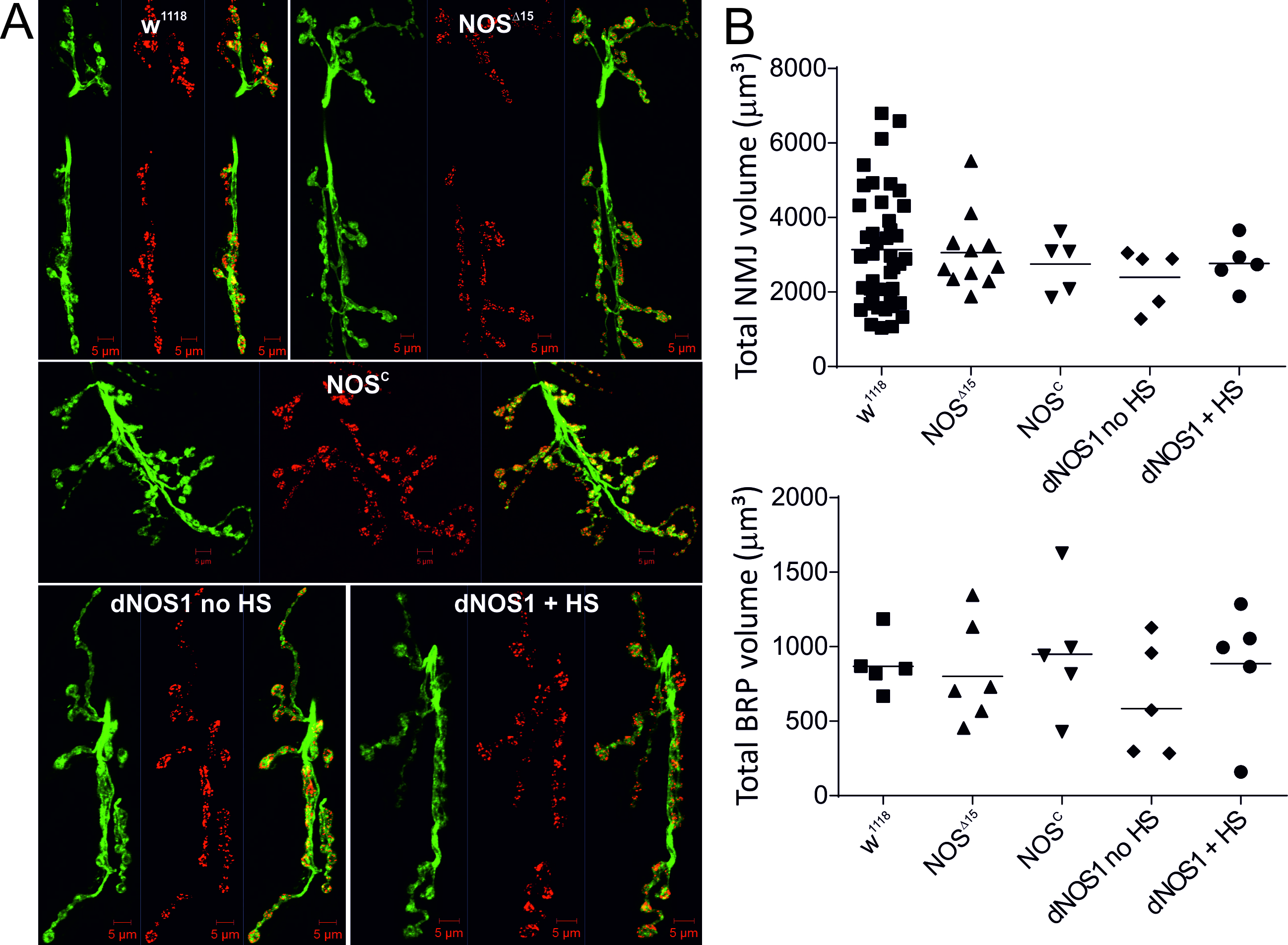
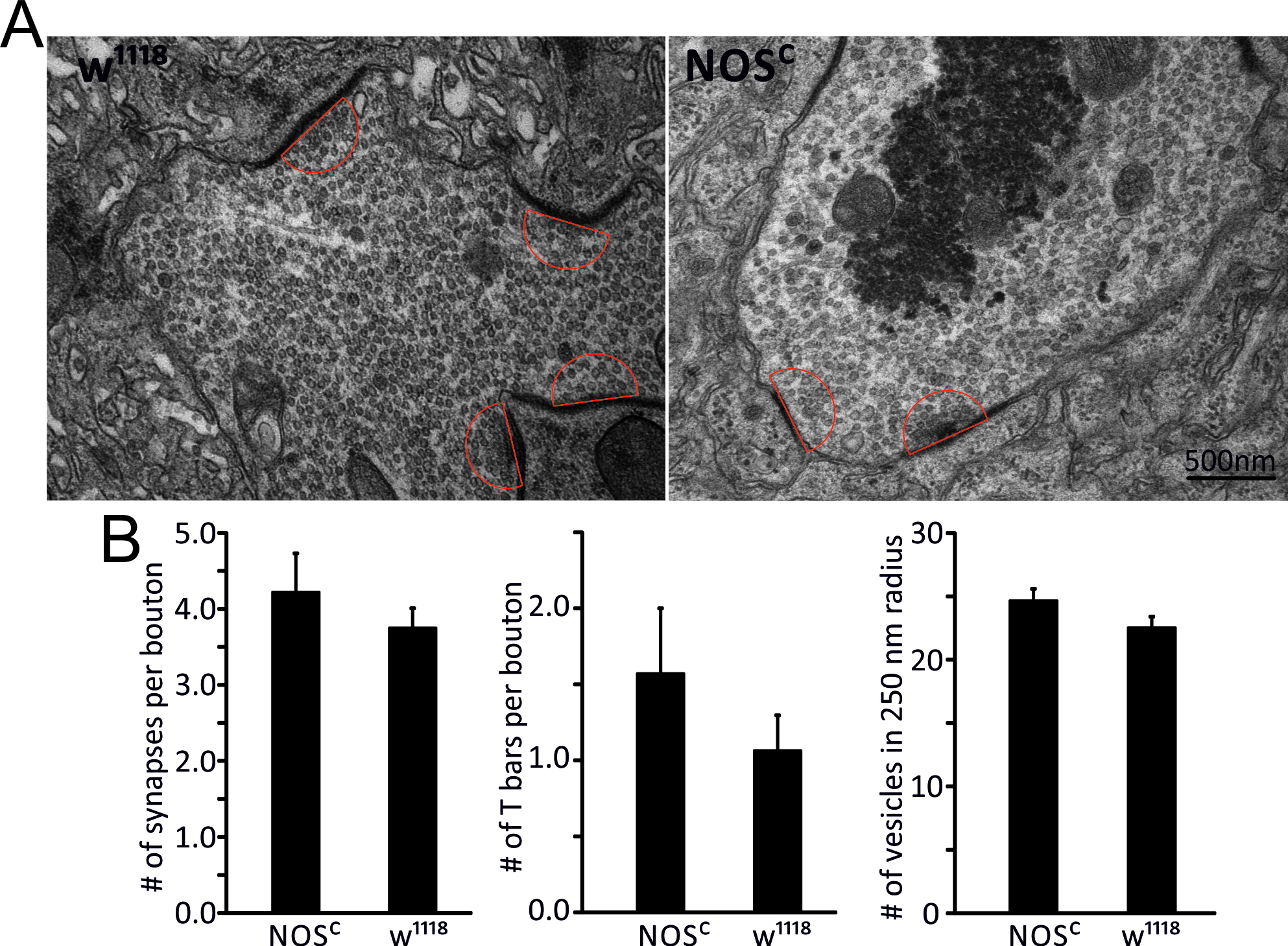
As NO has been reported to not only affect acute signalling pathways but can also be involved in developmental aspects of neuronal activity (Comes et al., 2007; Lacin et al., 2014) including its contributions to aging of the brain (Jung et al., 2012) we tested whether our observed effects in NOSC / NOS∆15 larvae could be caused by alterations in NMJ morphology. We found no evidence for changes in either total volume of NMJs (HRP labelling) or the total volume of BRP signals as a measure of BRP expression levels at the 3rd instar larvae of the mutant lines (Fig. 5) consistent with previous reports (Yakubovich et al., 2010) and suggesting that the observed nitrergic effects are not caused by developmental changes of the NMJ. To further explore the possibility, that genetic reduction of NOS activity caused ultrastructural changes in vesicle numbers or active zones (AZ) we performed electron microscopy experiments and measured the number of vesicles within a 250 nm semicircle around the AZ in 1b boutons of the NMJs. As we observed no difference in NMJ morphology, we only focused the ultrastructural analysis on two genotypes, w1118 and NOSC to test the developmental effects of lack of NO signalling. This data confirmed that the number of AZs, including T-bar structures, and the number of vesicles located within a 250 nm semicircle are not different between the two genotypes (Fig. 6). Thus our data imply that the observed effects of NO on glutamatergic release are likely to be due to direct nitrergic actions on the release rather than being mediated by developmental effects of NO on synapse structures.
Together, our study provides a comparative data set which illustrates the effects of NOS activity on synaptic physiology and morphology. Interestingly, enhanced NO exposure induced by enhanced dNOS activity is associated with a reduction in glutamatergic transmission at the larval NMJ, whereas reduced NOS activity results in enhanced release.
Figure 5. Changes in NOS activity do not cause developmental effects of NMJ morphology. A, Confocal maximal projection images of NMJs of indicated genotypes with HRP (green) and BRP (nc82, bruchpilot, red) labelling. B, Total NMJ volume and BRP volume were calculated form z-stack confocal images, analysis of NMJ and BRP volumes revealed no difference between genotypes, n=36-5 NMJs, p>0.05, ANOVA with post hoc Tukey-Kramer was used for comparisons.
Discussion
Nitric Oxide regulates a great number of physiological and pathological signalling pathways in neuronal function. Many physiological actions of NO occur via the activation of the NO-sensitive receptor sGC which generates cGMP. The Drosophila NMJ shows activity-and Ca2+-dependent generation of cGMP signalling involving NOS activation (Shakiryanova & Levitan, 2008) and PKG-mediated modulation of oxidative stress responses (Caplan et al., 2013). This suggests functional relevance of the NO-cGMP-PKG cascade at the NMJ. Other activity and kinase-dependent regulatory mechanisms modulating synaptic responses include PKA-mediated presynaptic facilitation of neurotransmitter release (Davis et al., 1998; Steinert et al., 2006; Cho et al., 2015).
In this study, we wanted to establish how regulation of NO levels affects neurotransmitter release in a controlled environment using genetic modifications of endogenous NO release capacity at the well-characterised glutamatergic synapse, the Drosophila NMJ. We employed genetic mutants which either exhibit reduced (NOSC / NOS∆15 ) or enhanced NOS activity (dNOS1+HS) and compared the synaptic responses to wild-type w1118 NMJs. Our data illustrated that by reducing endogenous NOS activity, the synaptic response increases with respect to spontaneous and evoked vesicular release. In contrast, when enhancing NOS activity, we detected a reduced release of vesicles. Spontaneous release frequency is increased in NOS ‘null’ larvae; however, enhanced nitrergic activity only induces a tendency to reduce spontaneous release relative to wild-type. This non-significant response could be the consequence of a subtle NOS activity in wild-type which already partially supresses release. More drastic effects of activated NO signalling were observed on evoked release. Quantal content as well as available vesicle pool sizes were strongly reduced following enhanced NOS activity suggesting a robust inhibitory action of NO on evoked release.
Importantly, neuronal development could potentially be responsible for the observed physiological effects in the genetic mutants. It has been reported that NO can affect neuronal development by fine-tuning axon degeneration and regrowth in Drosophila mushroom bodies (Rabinovich et al., 2016). When assessing NMJ morphology, we did not detect any morphological changes of NMJs including the release sites, total NMJ volume or ultra-structural changes within boutons which could account for the changes in transmitter release. Thus, our data strongly suggest that the observed effects on neurotransmitter release are due to acute functional modulations of transmitter release induced by nitrergic signalling.
Together, we have shown that NO reduces the transmitter release at the NMJ which could potentially involve the cGMP signalling cascade. However, it is also well established that NO can lead to thiol S-nitrosylation of cysteine and 3-nitrotyrosination of tyrosine residues of proteins, two NO-mediated post-translational modifications. These modifications can have major impacts on neuronal function and synaptic release (Knott & Bossy-Wetzel, 2009; Bradley & Steinert, 2016). There is discrepancy in the literature regarding the effects of NO on neuronal activity, mainly due to the variable conditions and concentrations of NO applied. S-NO formation of various proteins can lead to opposite effects on neurotransmitter release. Postsynaptic proteins that are negatively affected by S-nitrosylation include mammalian NMDA receptors (NR2A and NR1) (Takahashi et al., 2007) which mediate excitatory neurotransmission and are essential for learning and memory. Nitrosylation of scaffolding proteins postsynaptic density-95 (PSD-95) decreases glutamate receptor clustering at synaptic sites (Ho et al., 2011), further compromising neuronal function. Presynaptic proteins are nitrosylated (e.g. syntaxin) (Wiseman et al., 2011) which reduces exocytosis, all of which can exacerbate detrimental effects of NO on neurotransmission as seen in neurodegenerative conditions. At this point, further experiments are required to investigate the underlying pathways for the observed nitrergic effects in our study which could potentially be mediated via cGMP or PTM signalling at the synapse.
Figure 6. Changes in NOS activity do not cause developmental ultrastructural effects. A, Representative electron microscopy images of 1b boutons from each genotype with red semicircles indicating the area for vesicle counts. B, mean values for the number of synapses (AZ), number of T-bars and number of vesicles within a 250 nm semicircle from the centre of the AZ for each genotype, n=10-15 boutons, p>0.05, Student’s t-test. Data denote Mean ± SEM.
Acknowledgments
Fly stocks were kindly provided by Boris Kuzin, Grigori Enikolopov and Patrick O'Farrell. The University of Iowa Developmental Studies Hybridoma Bank (DSHB) provided essential reagents. Many thanks to Dr David Read (MRC Toxicology Unit) for support in confocal microscopy and Dr Amanda Wyatt-Steinert for valuable support and comments on the manuscript.
Funding
This work was supported by the Medical Research Council, UK.
Conflict of Interests Statement
The authors declare no competing financial interests.
List of Abbreviations
active zone- AZ
central nervous system- CNS
cysteine- Cys
Drosophila NOS- dNOS
nitric oxide- NO
endothelium-derived relaxing factor- EDRF
heat shock- HS
neuromuscular junction- NMJ
nitric oxide synthases- NOS
post-translational modifications- PTM
quantal content- QC
sodium nitroprusside- SNP
soluble guanylyl cyclase- sGC
S-Nitrosothiols- S-NO
SV-synaptic vesicles
wild-type- WT
| Attachment | Size |
|---|---|
| 3.06 MB |
Fly stocks were kindly provided by Boris Kuzin, Grigori Enikolopov and Patrick O'Farrell. The University of Iowa Developmental Studies Hybridoma Bank (DSHB) provided essential reagents. Many thanks to Dr David Read (MRC Toxicology Unit) for support in confocal microscopy and Dr Amanda Wyatt-Steinert for valuable support and comments on the manuscript.
Alderton WK, Cooper CE & Knowles RG. (2001). Nitric oxide synthases: structure, function and inhibition. The Biochemical journal 357, 593-615.
Bradley SA & Steinert JR. (2016). Nitric Oxide-Mediated Posttranslational Modifications: Impacts at the Synapse. Oxidative medicine and cellular longevity 2016, 5681036.
Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC & Bredt DS. (1996). Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84, 757-767.
Caplan SL, Milton SL & Dawson-Scully K. (2013). A cGMP-dependent protein kinase (PKG) controls synaptic transmission tolerance to acute oxidative stress at the Drosophila larval neuromuscular junction. Journal of neurophysiology 109, 649-658.
Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z & Lipton SA. (2009). S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science 324, 102-105.
Cho RW, Buhl LK, Volfson D, Tran A, Li F, Akbergenova Y & Littleton JT. (2015). Phosphorylation of Complexin by PKA Regulates Activity-Dependent Spontaneous Neurotransmitter Release and Structural Synaptic Plasticity. Neuron 88, 749-761.
Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HS & Lipton SA. (2000). Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nature neuroscience 3, 15-21.
Comes S, Locascio A, Silvestre F, d'Ischia M, Russo GL, Tosti E, Branno M & Palumbo A. (2007). Regulatory roles of nitric oxide during larval development and metamorphosis in Ciona intestinalis. Developmental biology 306, 772-784.
Davis GW, DiAntonio A, Petersen SA & Goodman CS. (1998). Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron 20, 305-315.
Di Stasi AM, Mallozzi C, Macchia G, Maura G, Petrucci TC & Minetti M. (2002). Peroxynitrite affects exocytosis and SNARE complex formation and induces tyrosine nitration of synaptic proteins. Journal of neurochemistry 82, 420-429.
Enikolopov G, Banerji J & Kuzin B. (1999). Nitric oxide and Drosophila development. Cell death and differentiation 6, 956-963.
Furchgott RF & Zawadzki JV. (1980). The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373-376.
Garthwaite J. (2016). From synaptically localized to volume transmission by nitric oxide. The Journal of physiology 594, 9-18.
Garthwaite J, Charles SL & Chess-Williams R. (1988). Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature 336, 385-388.
Hardingham N, Dachtler J & Fox K. (2013). The role of nitric oxide in pre-synaptic plasticity and homeostasis. Frontiers in cellular neuroscience 7, 190.
Ho GP, Selvakumar B, Mukai J, Hester LD, Wang Y, Gogos JA & Snyder SH. (2011). S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of PSD-95. Neuron 71, 131-141.
Jung J, Na C & Huh Y. (2012). Alterations in nitric oxide synthase in the aged CNS. Oxidative medicine and cellular longevity 2012, 718976.
Knott AB & Bossy-Wetzel E. (2009). Nitric oxide in health and disease of the nervous system. Antioxidants & redox signaling 11, 541-554.
Knowles RG & Moncada S. (1994). Nitric oxide synthases in mammals. The Biochemical journal 298 ( Pt 2), 249-258.
Kuromi H & Kidokoro Y. (2002). Selective replenishment of two vesicle pools depends on the source of Ca2+ at the Drosophila synapse. Neuron 35, 333-343.
Kuzin B, Roberts I, Peunova N & Enikolopov G. (1996). Nitric oxide regulates cell proliferation during Drosophila development. Cell 87, 639-649.
Lacin H, Rusch J, Yeh RT, Fujioka M, Wilson BA, Zhu Y, Robie AA, Mistry H, Wang T, Jaynes JB & Skeath JB. (2014). Genome-wide identification of Drosophila Hb9 targets reveals a pivotal role in directing the transcriptome within eight neuronal lineages, including activation of nitric oxide synthase and Fd59a/Fox-D. Developmental biology 388, 117-133.
Ozawa K, Whalen EJ, Nelson CD, Mu Y, Hess DT, Lefkowitz RJ & Stamler JS. (2008). S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Molecular cell 31, 395-405.
Postlethwaite M, Hennig MH, Steinert JR, Graham BP & Forsythe ID. (2007). Acceleration of AMPA receptor kinetics underlies temperature-dependent changes in synaptic strength at the rat calyx of Held. The Journal of physiology 579, 69-84.
Rabinovich D, Yaniv SP, Alyagor I & Schuldiner O. (2016). Nitric Oxide as a Switching Mechanism between Axon Degeneration and Regrowth during Developmental Remodeling. Cell 164, 170-182.
Regulski M, Stasiv Y, Tully T & Enikolopov G. (2004). Essential function of nitric oxide synthase in Drosophila. Current biology : CB 14, R881-882.
Regulski M & Tully T. (1995). Molecular and biochemical characterization of dNOS: a Drosophila Ca2+/calmodulin-dependent nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America 92, 9072-9076.
Robinson SW, Nugent ML, Dinsdale D & Steinert JR. (2014). Prion protein facilitates synaptic vesicle release by enhancing release probability. Human molecular genetics 23, 4581-4596.
Schneggenburger R, Meyer AC & Neher E. (1999). Released fraction and total size of a pool of immediately available transmitter quanta at a calyx synapse. Neuron 23, 399-409.
Shakiryanova D & Levitan ES. (2008). Prolonged presynaptic posttetanic cyclic GMP signaling in Drosophila motoneurons. Proceedings of the National Academy of Sciences of the United States of America 105, 13610-13613.
Singel DJ & Stamler JS. (2005). Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annual review of physiology 67, 99-145.
Stasiv Y, Kuzin B, Regulski M, Tully T & Enikolopov G. (2004). Regulation of multimers via truncated isoforms: a novel mechanism to control nitric-oxide signaling. Genes & development 18, 1812-1823.
Stasiv Y, Regulski M, Kuzin B, Tully T & Enikolopov G. (2001). The Drosophila nitric-oxide synthase gene (dNOS) encodes a family of proteins that can modulate NOS activity by acting as dominant negative regulators. The Journal of biological chemistry 276, 42241-42251.
Steinert JR, Kuromi H, Hellwig A, Knirr M, Wyatt AW, Kidokoro Y & Schuster CM. (2006). Experience-dependent formation and recruitment of large vesicles from reserve pool. Neuron 50, 723-733.
Stewart BA, Atwood HL, Renger JJ, Wang J & Wu CF. (1994). Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. Journal of comparative physiology A, Sensory, neural, and behavioral physiology 175, 179-191.
Takahashi H, Shin Y, Cho SJ, Zago WM, Nakamura T, Gu Z, Ma Y, Furukawa H, Liddington R, Zhang D, Tong G, Chen HS & Lipton SA. (2007). Hypoxia enhances S-nitrosylation-mediated NMDA receptor inhibition via a thiol oxygen sensor motif. Neuron 53, 53-64.
Weyhersmuller A, Hallermann S, Wagner N & Eilers J. (2011). Rapid active zone remodeling during synaptic plasticity. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 6041-6052.
Wildemann B & Bicker G. (1999). Developmental expression of nitric oxide/cyclic GMP synthesizing cells in the nervous system of Drosophila melanogaster. Journal of neurobiology 38, 1-15.
Wiseman DA, Kalwat MA & Thurmond DC. (2011). Stimulus-induced S-nitrosylation of Syntaxin 4 impacts insulin granule exocytosis. The Journal of biological chemistry 286, 16344-16354.
Yakubovich N, Silva EA & O'Farrell PH. (2010). Nitric oxide synthase is not essential for Drosophila development. Current biology : CB 20, R141-142.