Astrocytes are now recognised as important contributors to synaptic transmission control. Dopamine is a key neuromodulator in the mammalian brain and establishing the potential extent of its actions involving astrocytes is vital to our overall understanding of brain function. Astrocyte membranes can express receptors for dopamine, as well as dopamine transporters, but the full effects of dopamine on astrocytic physiology are still uncertain and its mode of action controversial. Here we overview the developing field of astrocyte-dopamine interaction, focusing on how dopamine affects the pre-eminent astrocytic intracellular signalling messenger – Ca2+ – and the available evidence for astrocyte-mediated effects of dopamine on neurons. We then discuss some of the methodological issues that need to be addressed to help move the field forward.
Introduction
The study of astrocytes has undergone a renaissance over the last three decades. With the advent of fluorescent Ca2+ imaging technology and increasingly advanced tools for genetic manipulation, astrocytes have revealed multifarious and dynamic intracellular Ca2+ activity (Rusakov et al., 2014, Volterra et al., 2014), as well as changes in other intracellular signalling pathways (see (Verkhratsky A, 2007) for detailed review), in response to common neurotransmitters (Shao and McCarthy, 1995, Cornell-Bell et al., 1990). Astrocytes are now considered important players in the machinery of the synapse – the ‘tripartite synapse’ – and much recent work has gone into how they can, in turn, modulate and control neuronal activity (Henneberger et al., 2010, Perea and Araque, 2007).
At this exciting time for astroglial research, it is unsurprising that increasing work is going into elucidating any involvement of astrocytes in the brain’s dopaminergic system. The monoamine dopamine is a major modulatory neurotransmitter in the CNS, implicated in reward processing, decision-making and action initiation and termination (Iversen LL, 2010). Various disorders – Parkinson’s disease, schizophrenia and ADHD amongst others – are hypothesised to be due, at least in part, to a malfunction of the dopaminergic system (for a detailed review see (Iversen LL, 2010, Nutt et al., 2015, Leyton and Vezina, 2014, Vaughan and Foster, 2013).
Astrocytes are widely reported to express dopaminergic receptors, and plasma membrane proteins capable of dopamine transport (Pelton et al., 1981, Vermeulen et al., 1994, Khan et al., 2001, Inazu et al., 2003). Activation of astrocytic dopamine receptors has been linked to changes in intracellular cyclic AMP (cAMP), free Ca2+ and αβcrystallin, amongst other signalling molecules (Zanassi et al., 1999, Shao et al., 2013, Parpura and Haydon, 2000), as has the metabolism of dopamine itself within the astrocyte soma after uptake. Cytoplasmic free Ca2+ is considered the pre-eminent astrocytic intracellular signalling molecule (Rusakov, 2015) and various studies have reported dopamine-induced astrocytic Ca2+ activity (Parpura and Haydon, 2000, Requardt et al., 2012, Khan et al., 2001), but the precise nature of these signals, their molecular pathways and possible functions are still unclear.
In this review we detail and discuss evidence for astrocytic ability to sense dopamine, the reported effects of dopamine on astrocytic physiology and the ways in which astrocytes has been suggested to go on to affect local neuronal activity. We also consider the technical challenges in the field and how divergent methodology could account for some of the current controversies in the literature.
Astrocytes sense dopamine and respond in a multitude of ways
Dopamine receptors (DARs), first identified in neurons and more recently in astrocytes, are of a metabotropic type and five different receptor subtypes have been identified (D1, D2, D3, D4 and D5).
D1 –Type Receptors
D1 receptors (D1Rs) and D5 receptors (D5Rs) are excitatory in neurons – hence they have been classified together, as the ‘D1-type receptors’– they appear to boost AMPA and NMDA receptor (Snyder et al., 1998, Flores-Hernandez et al., 2000) and L-type calcium-channel currents (Surmeier et al., 1995). Agonists binding to these receptors activate PKA via cyclic AMP (cAMP). cAMP is activated by adenyl cyclase (AC) via the g-protein αolf/αs subunit coupled to the intracellular domain of the receptor. There have been recent reports of a novel phosphatidylinositol (PI)-linked D1-like receptor (so called because of its high affinity to the selective D1-type receptor ligand SCH23390) in neurons and astrocytes, which couples to PLC through Gαq and can increase intracellular free Ca2+ (see below) (Liu et al., 2009, Ming et al., 2006). This receptor presents an intriguing way of stimulating the major Ca2+ signaling pathway in astrocytes through a molecular cascade traditionally associated with cAMP increase. Indeed, selective stimulation of this receptor through the agonist SKF83959 has been shown to trigger astrocytic calcium transients (Liu et al., 2009) in culture and concomitant ERK1/2 activation (Huang et al., 2012).
There is strong evidence from culture studies for expression of D1 and D5 receptors in rat astrocytes. From the striatum, autoradiographic dopamine receptor labelling has been reported (Hosli and Hosli, 1986), as has D1-type receptor mRNA expression (Zanassi et al., 1999, Miyazaki et al., 2004, Brito et al., 2004). Correspondingly, selective D1-type receptor stimulation with the specific agonists SKF38393 and SKF81297 has been found to raise intracellular astrocytic cAMP levels (Zanassi et al., 1999, Vermeulen et al., 1994, Requardt et al., 2010). Astrocytes cultured from the cortex also display cAMP rises in response to SKF38393, although they are not as pronounced (Zanassi et al., 1999). Cortical astrocytes stimulated with dopamine show D1/5R mediated intracellular NADH increase, via PKA, (Requardt et al., 2010) in situ and concomitant Ca2+ increases (Requardt et al., 2012) in vitro. 24h chronic stimulation with SKF39383 stimulates NGF and GDNF release from astrocytes (Ohta et al., 2010). See Figure 1 for a summary of proposed D1-type receptor mediated pathways.
Whereas the above studies come from rodent preparations, astrocytic D1-type receptors have also been reported in monkey and human cultured striatal astrocytes – with different receptor affinities compared to rat, indicating a possible species difference in astrocyte-dopamine interaction (Vermeulen et al., 1994).
D2 receptor
D2, D3 and D4 receptors are inhibitory in neurons – they inhibit AMPAR, NMDAR and L- type Ca2+ channel current (Iversen LL, 2010) – and are grouped together as ‘D2-type receptors’. They can also oligomerize with D1Rs (Lee et al., 2004, Hasbi et al., 2010), CB1Rs (Kearn et al., 2005) and A2ARs (Torvinen et al., 2004, Torvinen et al., 2005). D2-like receptors inhibit AC activation via αi/o G-proteins and activate PLC via Gβγ, increasing intracellular IP3 – an important excitatory pathway in astrocytes (Rusakov et al., 2011).
D2-type receptors have been localized on prefrontal cortical astrocytes in situ with electron microscopy in rat (Duffy et al., 2011), and human (Khan et al., 2001) samples, and D2-type receptor RNA has been found in rodent striatal astrocytes (Bal et al., 1994, Miyazaki et al., 2004). The D2 receptor agonist ropinerole (applied over varying time periods) stimulates NGF and GDNF secretion from cultured rat cortical astrocytes (Ohta et al., 2010) and selective D2R activation with quinpirole inhibits αcrystallin-mediated inflammation-associated astrogliosis in the prefrontal cortex of rats in vivo (Shao et al., 2013). In rat hippocampal astrocytes in situ, D2-like receptors mediate apomorphine (a non-specific dopamine receptor agonist) induced S100B secretion (Nardin et al., 2011). In cultured rodent prefrontal cortical astrocytes, the D2 agonist quinpirole has been reported to marginally increase Ca2+ (see below) (Khan et al., 2001). See Figure 1 for a summary of proposed D2-type receptor mediated pathways.
Dopamine stimulation triggers protein tyrosine phosphorylation in cultured human glioma cells in a D2R-dependent manner (Luo et al., 1999), D2Rs are also upregulated in glioma cells and have been shown to be pro-proliferative (Li et al., 2014).
Non-specific DAR activation
Many studies have recorded changes in astrocytic activity in response to non-specific dopaminergic stimulation with both exogenously applied agonists and endogenously (Parpura and Haydon, 2000, Reuss and Unsicker, 2001, Li et al., 2006). Striatal astrocytic FGF-2 expression in response to apomorphine has been reported to be both D1 and D2 receptor dependent (Li et al., 2006). It is interesting to note that functional hetero-oligomerization of D1 and D2 receptors has been found in cells co-expressing both receptor types in striatal neurons of the rat brain (Hasbi et al., 2010) – activation of this hetero-oligomer increases intracellular Ca2+ (in HEK cells) in a PLC- dependent fashion (Lee et al., 2004), via the Gq/11 protein (Rashid et al., 2007). Given the importance of the Ca2+ signaling pathway in astrocytes, it would be interesting to see if this hetero-oligomer mediated dopaminergic signaling in astrocytes.
A recent study has also found that stimulation of cultured cortical rat astrocytes with dopamine leads to an increase in TNFα secretion via toll-like receptor 4 (TLR4) activation (Ding et al., 2015) – raising the prospect of an entirely new, DAR-insensitive form of astrocytic dopaminergic signaling. Dopaminergic modulation (after chronic application of dopamine) of astrocytic Ca2+ response to NMDA challenge has also been reported (Ding et al., 2014) in rodent cortical astrocyte cultures, as has astrocytic GLT-1 upregulation in response to dopaminergic fibre dennervation in the rodent prefrontal cortex (Vollbrecht et al., 2014).
Dopamine uptake and metabolism
Dopamine is transported from the extracellular space across the plasma membrane into the cytosol via uptake1 system and uptake2 system (Koepsell et al., 2007). Uptake1 is faster, higher-affinity, presynaptic neuron-based transport whereas uptake2 is slower, lower-affinity, extraneuronal transport.
Uptake1 occurs primarily through the dopamine transporter (DAT), although the norepinephrine transporter (NET) can also transport dopamine (indeed the NET has greater affinity for dopamine than norepinephrine (Pacholczyk et al., 1991)). Both are a part of the family of Na+/Cl- dependent neurotransmitter transporters and transport two Na+ ions and one Cl- ion into the cytosol with each dopamine molecule (Torres et al., 2003), down the Na+ electrochemical gradient.
Classically the DAT and NET are considered to be only expressed on presynaptic neurons in situ (Lorang et al., 1994, Ciliax et al., 1995, Schroeter et al., 2000), but there is accumulating evidence that cultured astrocytes also express dopamine transport machinery (Pelton et al., 1981, Inazu et al., 1999a, Takeda et al., 2002, Schroeter et al., 2000).
Cultured astrocytes, taken from whole rat brain, express rapid Na+-dependent dopamine uptake1 machinery (Pelton et al., 1981). It has been suggested that astrocytic DA uptake1 is mediated not by DAT, but by NET: DAT-specific blockers inhibit astrocytic dopamine uptake far less than NET-specific ones (Takeda et al., 2002, Inazu et al., 1999a). Evidence for NET expression in astrocytes comes from radioactive tracer uptake studies in cultured rat neocortical astrocytes (Inazu et al., 1999a, Takeda et al., 2002, Inazu et al., 2003) and mRNA expression in cultured rat spinal astrocytes (Schroeter et al., 2000). There are even conflicting reports over whether astrocytes even express DAT (Takeda et al., 2002) or not (Kittel-Schneider et al., 2012).
Uptake2 is mediated by organic cation transporter (OCT) and plasma membrane monoamine transporter (PMAT), independently of Na+ and Cl- and is recognised to play an important role in dopamine clearance from the synapse (Baganz et al., 2008, Bacq et al., 2012, Ordway GA, 2007). Human astroglioma cells are reported to transport dopamine via uptake2 in a Na+-independent manner (Russ et al., 1996), through OCT3 and PMAT – for which they express the mRNA (Naganuma et al., 2014).
So far, there is currently no consistent in situ evidence to support normal astrocytic expression of either DAT or NET. The number of studies reporting DAT or NET transport in astrocytic culture says as much about the remarkable plasticity of astrocytes as it does about their involvement in dopamine transport. Although it is clear astrocytes can selectively express dopamine uptake mechanisms (expression which is itself plastic through FGF and EGF (Inazu et al., 1999b)) and that DAT can be expressed in pathologically stimulated astrocytes in vivo – after treatment with L-DOPA in 6-OHDA parkinsonian model rats (Asanuma et al., 2014) – whether they do under normal conditions in an intact brain remains to be ascertained.
Astrocytes are able to metabolise dopamine intracellularly, as they express both catechol-O-methyl transferase (COMT) and mono-amine oxidase (MAO) (Pelton et al., 1981, Hansson and Sellstrom, 1983). COMT catalyzes the transfer of a methyl group from S-adenosyl-methionine to a hydroxyl group on the catechol nucleus of dopamine (Chen et al., 2004). MAO deaminates dopamine – a reaction that produces H2O2 via FAD (Youdim and Bakhle, 2006).
The H2O2 produced by the breakdown of dopamine by MAO has been linked to an increase in rat cortical astrocyte intracellular calcium (Vaarmann et al., 2010) in culture. H2O2 activates lipid peroxidation which activates PLC, causing the release of IP3 which increases the likelihood of Ca2+ release from intracellular stores (Vaarmann et al., 2010) (Figure 2A).
Antipsychotics
The pathogenesis of schizophrenia has been linked to changes in astrocytes (Schnieder and Dwork, 2011). Antipsychotics used to treat schizophrenia, are known to act through blockade of D2-type receptors (Carlsson and Lindqvist, 1963) – through which they exert some of their antipsychotic effects (Meltzer and McGurk, 1999). Clozapine, an atypical neuroleptic, is one such drug – it attenuates schizophrenia-induced GFAP expression in astrocytes in a rat model of schizophrenia (Gomes et al., 2015).
Interestingly, clozapine appears to directly affect astrocytes in addition to any effects it has by blocking DARs. After clozapine incubation, cultured rat astrocytes (mixed cortex and striatum) show decreased Ca2+ response to dopamine challenge (Reuss and Unsicker, 2001) and enhanced d-serine and l-glutamate release (Tanahashi et al., 2012). Cultured glioma cells show enhanced S100β release after clozapine incubation (Steiner et al., 2010), and clozapine inhibits proteomic change in MK801-treated (a pharmacological model of schizophrenic physiology) glioma cells in culture (Martins-de-Souza et al., 2011).This clozapine-mediated astrocytic activity is very interesting, but so far no possible mechanism of action has been proposed for clozapine’s effects on astrocytes. It presents another interesting locus of interaction between astrocytes and dopamine – one that merits further study.
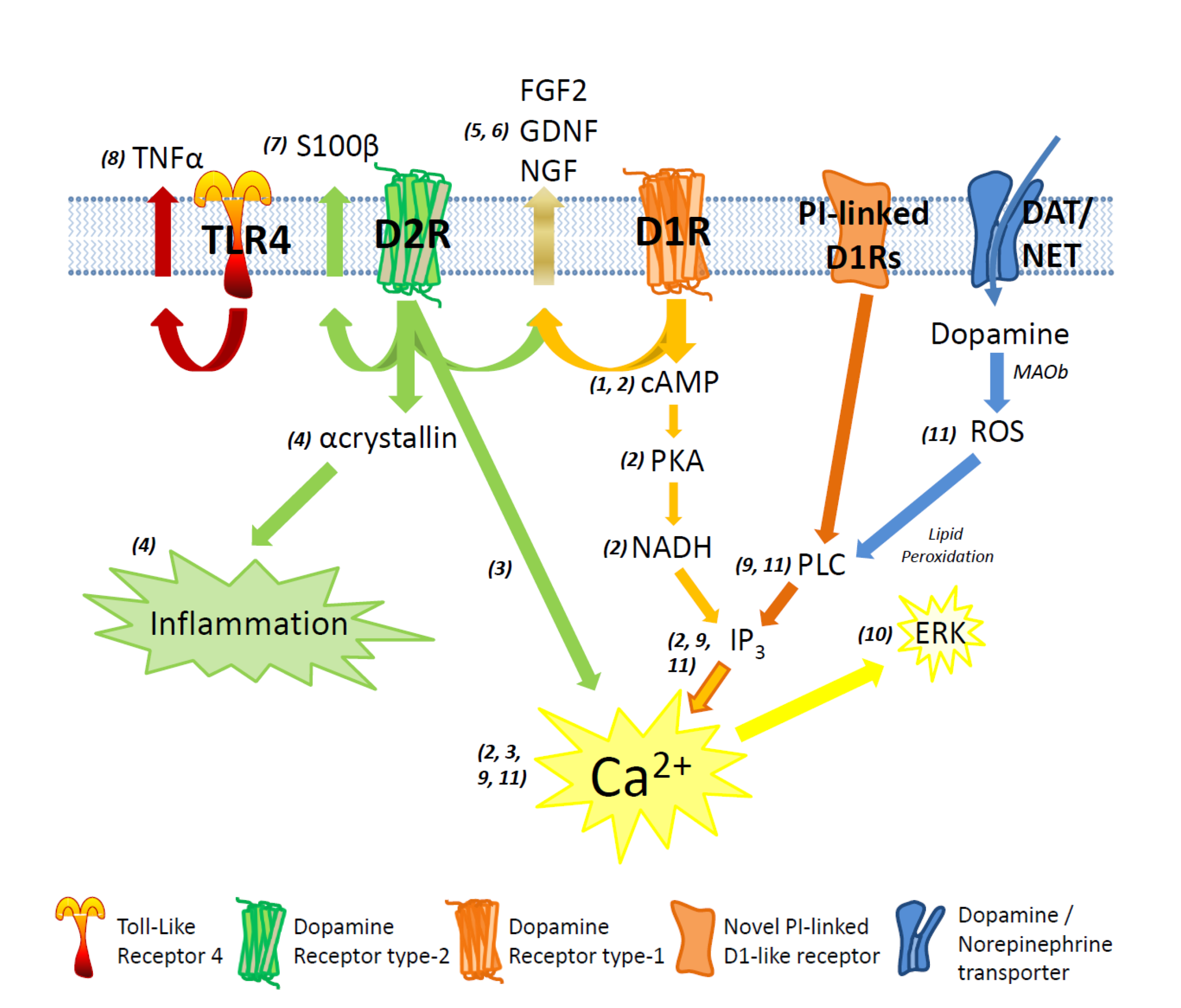
Figure 1. Putative pathways for dopaminergic signalling in astrocytes. D1-type receptor (D1R) stimulation increases cytoplasmic cyclic AMP (cAMP), leading to an increase in Protein Kinase A (PKA), NADH, IP3 and Ca2+. D2-type receptor (D2R) stimulation leads to an increase in Ca2+, S100β secretion, and also inflammatory response mediated via αcrystallin. Joint activation of D1Rs and D2Rs causes secretion of Fibroblast Growth Factor 2 (FGF2), Glial-Derived Neurotrophic Factor (GDNF) and Nerve-Growth Factor (NGF). Activation of novel phosphatidyl-inositol-linked D1R stimulates intracellular Ca2+ rise via PLC and IP3 increase. Metabolism of dopamine via Monoamine Oxidase B (MAOb) creates Reactive Oxygen Species (ROS) and activates the PLC/IP3/Ca2+ pathway. Ca2+ increase triggered through novel PI-linked D1R leads to ERK expression.
Astrocytic calcium signalling and dopamine
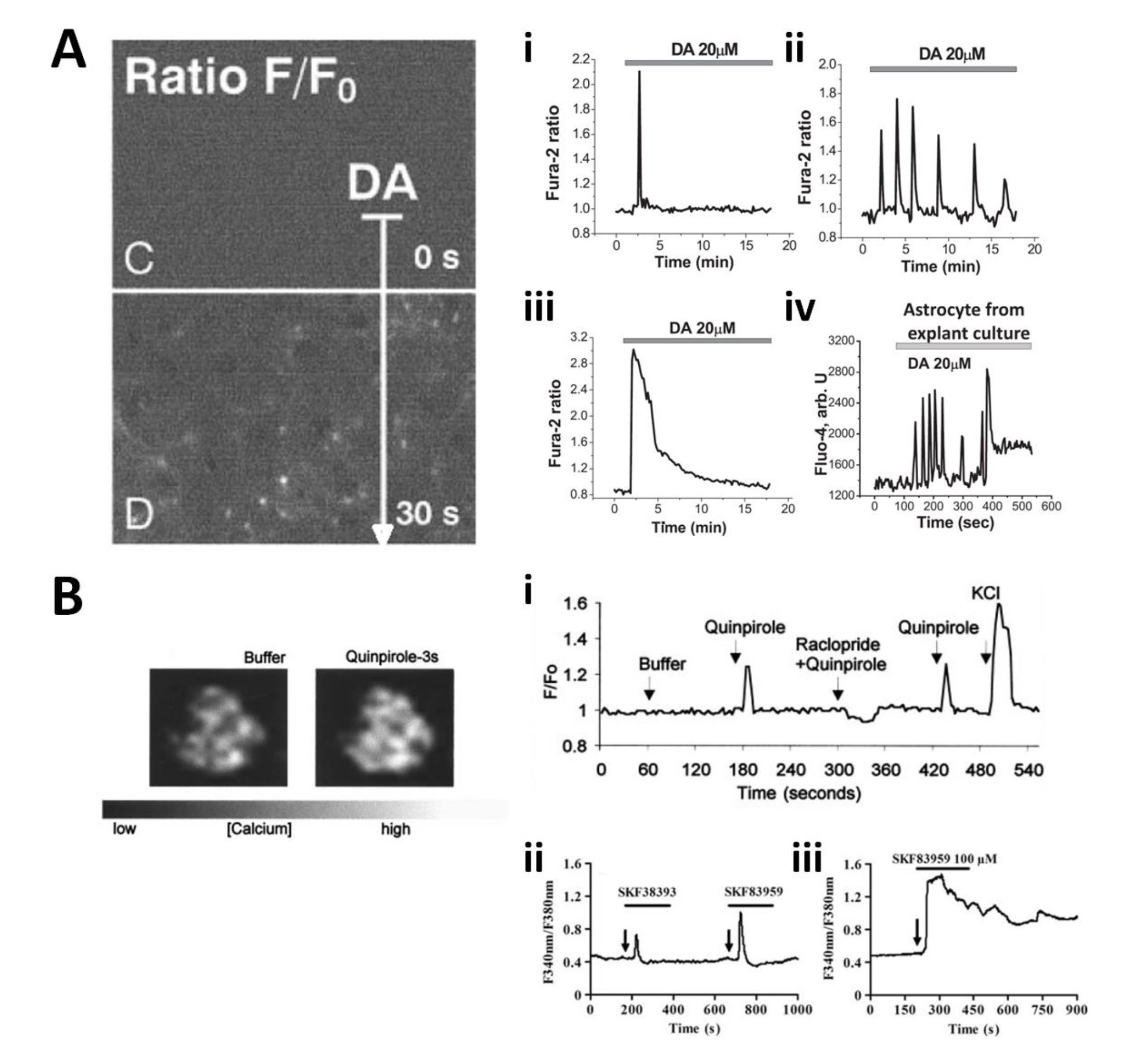
Much work has gone into interrogating astrocytic Ca2+ signalling, given the complicated intracellular astrocytic Ca2+ dynamics witnessed in vitro (Cornell-Bell et al., 1990), in situ (Di Castro et al., 2011) and in vivo (Gourine et al., 2010), in response to neurotransmitter simulation (Shao and McCarthy, 1995), neuronal activity (Navarrete and Araque, 2010) and physiological stimuli (Gourine et al., 2010). Astrocytes also display spontaneous Ca2+ activity that can be modulated (Di Castro et al., 2011, Requardt et al., 2012). Astrocytic Ca2+ signalling can trigger release of neuroactive and glioactive gliotransmitters from astrocytes (Perea and Araque, 2007, Gourine et al., 2010), through exocytosis (Perea and Araque, 2007) and hemichannel opening (Montero and Orellana, 2015), and the debate still rages as to the extent of their involvement in information processing in the brain (Rusakov et al., 2011). Hence, astrocytic Ca2+ response to dopamine is of particular interest; however until now the problem has only been addressed using experiments in vitro. Rodent cortical astrocytes in culture are sensitive to concentrations of dopamine above 5µM, and respond through single sharp Ca2+ single transients, repeated Ca2+ transients or broader Ca2+ increases (Figure 2A) (Vaarmann et al., 2010, Parpura and Haydon, 2000, Reuss and Unsicker, 2001). The amplitude and frequency of these Ca2+ transients increases with dopamine concentration (Requardt et al., 2012) and have been reported to reach peak concentrations of 400 to 2500nM (Vaarmann et al., 2010, Parpura and Haydon, 2000). It has not been possible it appears to predict or control the precise kind of Ca2+ activity that dopamine application will induce in cultured astrocytes (Figure 2A) (unlike, say, action potential shape triggered by a constant depolarization in neurons). However, inhibition of dopamine-induced Ca2+ elevations has been reported after blockade of MAOb (Vaarmann et al., 2010), or following suppression of D1Rs and NADH activity (Requardt et al., 2012). Slight dopamine-induced Ca2+ increases (~20% above baseline) had previously been reported following D2R stimulation in rodent cortical astrocytes (Khan et al., 2001) (Figure 2Bi), as had dose-dependent Ca2+ increases following selective D1-like receptor stimulation in rat cortical astrocytes (Liu et al., 2009) (Figure 2Bii, iii). Although none of the above studies agree on the overall nature of the DA signalling pathway, they consistently reported that astrocytic dopaminergic Ca2+ signals are mediated by IP3 release into the cytosol – a well-documented astrocyte Ca2+ signalling pathway.
Figure 2. Dopamine-induced astroglial Ca2+ signals. A. Picture: cultured rodent cortical/striatal astrocytes display Ca2+ increases – measured with fluo-3 AM – fluorescence upon challenge with 100µM dopamine (adapted from Reuss and Unsicker, 2001). A i-iii. Example traces of differing Ca2+ responses – measured with Fura-2 – to an identical stimulus, 20µM dopamine, in primary cultured cortical astrocytes. A iv. Cortical astrocytes from explant cultures display different Ca2+ activity – measured with fluo-4 AM (adapted from Vaarmann et al. 2010). B. Picture: cultured rodent cortical astrocytes respond to 150µM of the D2R agonist quinpirole with an increase in Ca2+ measured with fluo-3 – in left panel, compared to baseline in right panel. B i. Example trace of cortical astrocyte response to 150µM quinpirole (adapted from Kahn et al. 2001). B ii, iii. Stimulation of rodent cortical astrocyte Ca2+ - measured with fura-2 AM – with the novel PI-linked D1-like receptor agonist SKF83959 at 25µM (ii) and 100µM (iii) (adapted from Liu et al. 2009).
Astrocyte-mediated effects of dopamine on neurons
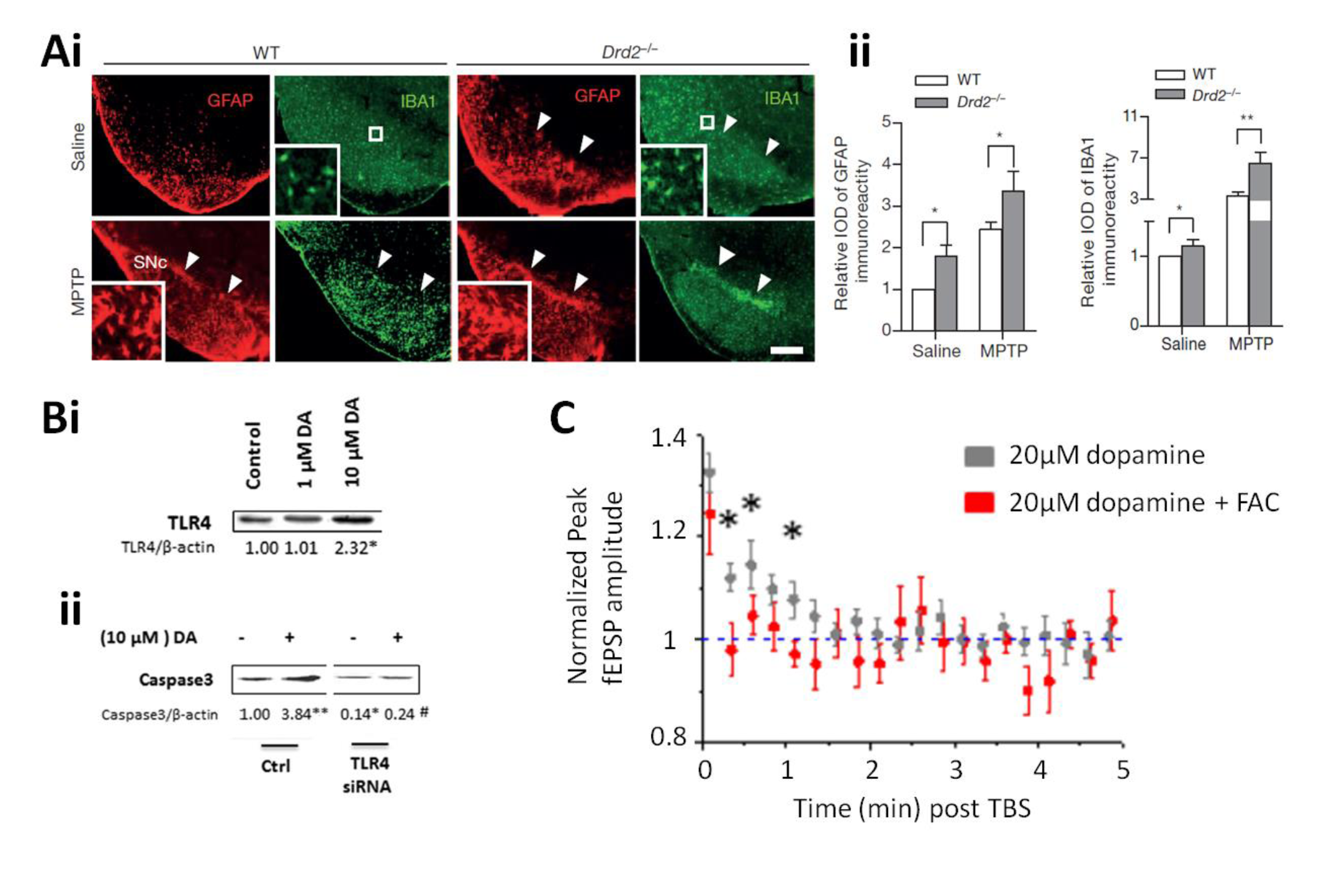
An apparent link between dopamine-induced Ca2+ elevation and Ca2+-dependent gliotransmitter exocytosis, GDNF, NGF, TNFα and inflammatory response (Parpura and Haydon, 2000, Ohta et al., 2010, Shao et al., 2013, Ding et al., 2015) raises the exciting theoretical possibility of dopamine-induced astrocytic communication with neurons . However, only two recent studies have shown this in a relatively direct manner. Shao et al. (2013) found that astrocytic DRD2 null mice expressed increased markers for astrocytic inflammatory activation (Figure 3A), which in turn lead to increased vulnerability of neurons to stress-induced apoptosis in the midbrain in vivo. They suggest that tonic activation of astrocytic DRD2s in the healthy brain regulates immune response via αβ-crystallin – a previously unreported signalling pathway. Ding et al. (2015) find that dopamine hyperstimulation (10µM for 24h) of neuron/astrocyte co-cultures triggers neuronal cell death mediated by astrocytic release of TNFα via dopaminergic stimulation of TLR4 (Figure 3B). Astrocytes are known to release TNFα in both healthy and pathological situations (Santello and Volterra, 2012), but this dopamine/TLR4 pathway represents a novel mode of TNFα stimulation. Interestingly, TNFα is one of the few inflammatory pathways not upregulated in DRD2 null mice (Shao et al., 2013) – adding weight to the idea that TNFα secretion is under control of TLR4.
So far, the only direct evidence in the literature of a dopaminergic effect on neurons via astrocytes comes from studies of astrocyte-mediated neurotoxicity (Shao et al., 2013, Ding et al., 2015). There is no literature directly examining any putative dopaminergic regulation of normal brain function, such as synaptic plasticity or homeostasis – although astrocytes are thought to be integral to these processes (Rusakov et al., 2014, Henneberger et al., 2010). In our recent attempt to detect such phenomena (Jennings, 2014) we examined putative astrocytic involvement in the dopaminergic inhibition of post-tetanic potentiation (PTP) at the perforant-path – CA1 synapse in the Stratum Lacunosum-Moleculare in the hippocampus, the prominent inhibition phenomenon reported earlier (Otmakhova and Lisman, 1999) . The effects were not conspicuous: PTP was even more attenuated in the presence of dopamine (20µM) after glial poisoning with fluoroacetate (FAC) (Figure 3C). Accurate interpretation of such results could be complicated, partly down to the difficulty of isolating a purely astrocytic response to a physiological dopamine stimulus in organised brain tissue and recording any subsequent, potentially subtle, effects on neurons. Here perhaps lies one of the principal empirical obstacles in dissecting the physiological significance of dopamine-dependent signalling in astroglia.
Figure 3. Dopaminergic effects on neurons through astrocytes. A i. DRD2-null mice – left panel – show greater inflammatory response, as measured by GFAP and IBA1 expression, in midbrain astrocytes in avivo after both saline and MPTP injections. A ii. Summary of data from (Ai) (adapted from Shao et al. 2013). B i. Cortical astrocytes co-cultured with neurons express increased TLR4 upon stimulation with 10µM dopamine. B ii. Neurons co-cultured with cortical astrocytes stimulated with 10µM dopamine express caspase3, but do not when astrocytic TLR4 has been knocked down with siRNA (adapted from Ding et al. 2015). C. Post-tetanic potentiation in the Stratum Lacunosum-Moleculare in the presence of 20µM dopamine is attenuated after pre-incubation with the glial poison fluoroacetate (FAC) (adapted from Jennings, 2014).
Methodological issues
Much of the existing literature describing the mode of astrocytic response to dopamine is at odds, but there are some key experimental factors that may account for the variability of the findings in the literature.
Firstly, with the exception of cytoplasmic free Ca2+, it is still unsure what the major output activity is in astroglia and hence what pathways to examine, and their relative importance to overall astrocyte physiology in the brain. When considering Ca2+ activity, astrocytes do not seem to show stereotyped responses to identical stimuli (this is certainly true for dopaminergic stimuli). In addition to precluding the signal averaging and hence recording noise reduction, this feature makes it difficult to know what parts of the Ca2+ signal are carrying information (if any), whether the signal is transmitted through the cell and what downstream signalling pathways it activates.
Secondly, astrocytes are extremely reactive cells and change both morphologically and biochemically when stressed or placed in a new environment within hours (Shao and McCarthy, 1993, Kimelberg et al., 1997). Therefore studying them in culture or in situ may fundamentally change the nature of their recorded response (most of the studies reported in this review will keep cells in culture for up to a week before experimenting). It is interesting to examine Ca2+ recordings from astrocyte primary cultures in comparison to astrocyte explant cultures (acutely isolated from brain tissue), in response to dopamine (Vaarmann et al., 2010). There is a clear difference, not only in basal Ca2+ activity, but also in the nature of the highly variable response to dopamine application. When considering morphology, many physiologically relevant responses to dopamine may take place in the highly ramified astrocytic end-feet that form in organised tissue in situ; studying astrocytes in culture, where their shape is drastically simplified, risks missing many possible forms of dopamine-induced astrocyte activity.Thirdly, there are no standardized protocols for stimulating astrocytes with dopamine. Dopamine and DAR agonist application concentration ranges from 1-100µM, and stimulation can be acute (phasic) or chronic (up to 24h). Also, given that the various imaging techniques, mRNA expression measurements and excretion measurement s have different sensitivities, no two studies are easily comparable. It is also a major concern when inferring how astrocytes might interact with dopamine in more physiological situations – dopamine input to different brain regions can be acute or tonic and generally reaches far lower concentrations than those used in the above studies (Iversen LL, 2010), how much can they therefore tell us about what is happening the living brain?
Finally, astrocytes are ubiquitous throughout the different brain regions but it is still uncertain to what extent their respective populations differ. In contrast, somata of dopaminergic neurons are confined mainly to a small area of the midbrain – the Substantia Nigra and Ventral Tegmental Area – and project to spatially restricted areas of the basal ganglia, the frontal cortices and the limbic system (Iversen LL, 2010). As dopamine plays different roles in these functionally distinct areas, it is possible that any interaction uncovered between dopamine and astrocytes may be region-specific and thus not applicable across the whole astrocyte population. In this review we have been careful to state where different populations of astrocytes originate from – but given their extreme malleability in culture, it is unsure how much of their region-specific features they retain. Any reported regional heterogeneity in astrocyte-dopamine interaction (as in (Zanassi et al., 1999)), if robust, would be exceptionally interesting given that astrocytes are currently considered to form a relatively homogenous population.
Concluding remarks
We are only just beginning to uncover the interplay between the brain’s astrocyte population and one of its most powerful neuromodulators, dopamine. It is clear that astrocytes can express dopamine receptors and transporters and that dopamine application can trigger profound intracellular changes in astrocytes. However, for the most part, evidence as to the mechanism of dopamine’s actions is conflicting – due in part to inconsistent methodologies across the field, but also due to the inherent difficulty of studying both astrocytes and dopamine in the context of their action on neural networks. The study of astrocytic response to dopamine in situ has already begun and should go a long way to clarifying glial role in the dopaminergic modulation of neurotransmission. Once the major signalling pathways have been identified and clarified, the potential implications both therapeutic and for basic understanding of neuromodulation in the brain are enormous.
| Attachment | Size |
|---|---|
| 817.55 KB |
This work was supported by the Wellcome Trust Principal Fellowship, European Research Council Advanced Grant, Biology and Biotechnology Research Council (all UK), FP7 ITN EXTRABRAIN Marie Curie Action (European Commission), and Russian Science Foundation grant 15-14-30000.
ASANUMA M., MIYAZAKI I., MURAKAMI S., DIAZ-CORRALES F. J. & OGAWA N. (2014). Striatal astrocytes act as a reservoir for L-DOPA. PLoS One, 9, e106362.
BACQ A., BALASSE L., BIALA G., GUIARD B., GARDIER A. M., SCHINKEL A., LOUIS F., VIALOU V., MARTRES M. P., CHEVARIN C., HAMON M., GIROS B. & GAUTRON S. (2012). Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol Psychiatry, 17, 926-939.
BAGANZ N. L., HORTON R. E., CALDERON A. S., OWENS W. A., MUNN J. L., WATTS L. T., KOLDZIC-ZIVANOVIC N., JESKE N. A., KOEK W., TONEY G. M. & DAWS L. C. (2008). Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A, 105, 18976-18981.
BAL A., BACHELOT T., SAVASTA M., MANIER M., VERNA J. M., BENABID A. L. & FEUERSTEIN C. (1994). Evidence for dopamine D2 receptor mRNA expression by striatal astrocytes in culture: in situ hybridization and polymerase chain reaction studies. Brain Res Mol Brain Res, 23, 204-212.
BRITO V., BEYER C. & KUPPERS E. (2004). BDNF-dependent stimulation of dopamine D5 receptor expression in developing striatal astrocytes involves PI3-kinase signaling. Glia, 46, 284-295.
CARLSSON A. & LINDQVIST M. (1963). EFFECT OF CHLORPROMAZINE OR HALOPERIDOL ON FORMATION OF 3METHOXYTYRAMINE AND NORMETANEPHRINE IN MOUSE BRAIN. Acta Pharmacol Toxicol (Copenh), 20, 140-144.
CHEN J., LIPSKA B. K., HALIM N., MA Q. D., MATSUMOTO M., MELHEM S., KOLACHANA B. S., HYDE T. M., HERMAN M. M., APUD J., EGAN M. F., KLEINMAN J. E. & WEINBERGER D. R. (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet, 75, 807-821.
CILIAX B. J., HEILMAN C., DEMCHYSHYN L. L., PRISTUPA Z. B., INCE E., HERSCH S. M., NIZNIK H. B. & LEVEY A. I. (1995). The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci, 15, 1714-1723.
CORNELL-BELL A. H., FINKBEINER S. M., COOPER M. S. & SMITH S. J. (1990). Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science, 247, 470-473.
DI CASTRO M. A., CHUQUET J., LIAUDET N., BHAUKAURALLY K., SANTELLO M., BOUVIER D., TIRET P. & VOLTERRA A. (2011). Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci, 14, 1276-1284.
DING S., HUANG W., YE Y., YANG J., HU J., WANG X., LIU L., LU Q. & LIN Y. (2014). Elevated intracranial dopamine impairs the glutamatenitric oxidecyclic guanosine monophosphate pathway in cortical astrocytes in rats with minimal hepatic encephalopathy. Mol Med Rep, 10, 1215-1224.
DING S., WANG W., WANG X., LIANG Y., LIU L., YE Y., YANG J., GAO H. & ZHUGE Q. (2015). Dopamine Burden Triggers Neurodegeneration via Production and Release of TNF-alpha from Astrocytes in Minimal Hepatic Encephalopathy. Mol Neurobiol.
DUFFY A. M., FITZGERALD M. L., CHAN J., ROBINSON D. C., MILNER T. A., MACKIE K. & PICKEL V. M. (2011). Acetylcholine alpha7 nicotinic and dopamine D2 receptors are targeted to many of the same postsynaptic dendrites and astrocytes in the rodent prefrontal cortex. Synapse, 65, 1350-1367.
FLORES-HERNANDEZ J., HERNANDEZ S., SNYDER G. L., YAN Z., FIENBERG A. A., MOSS S. J., GREENGARD P. & SURMEIER D. J. (2000). D(1) dopamine receptor activation reduces GABA(A) receptor currents in neostriatal neurons through a PKA/DARPP-32/PP1 signaling cascade. J Neurophysiol, 83, 2996-3004.
GOMES F. V., LLORENTE R., DEL BEL E. A., VIVEROS M. P., LOPEZ-GALLARDO M. & GUIMARAES F. S. (2015). Decreased glial reactivity could be involved in the antipsychotic-like effect of cannabidiol. Schizophr Res, 164, 155-163.
GOURINE A. V., KASYMOV V., MARINA N., TANG F., FIGUEIREDO M. F., LANE S., TESCHEMACHER A. G., SPYER K. M., DEISSEROTH K. & KASPAROV S. (2010). Astrocytes control breathing through pH-dependent release of ATP. Science, 329, 571-575.
HANSSON E. & SELLSTROM A. (1983). MAO COMT, and GABA-T activities in primary astroglial cultures. J Neurochem, 40, 220-225.
HASBI A., O’DOWD B. F. & GEORGE S. R. (2010). Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Curr Opin Pharmacol, 10, 93-99.
HENNEBERGER C., PAPOUIN T., OLIET S. H. & RUSAKOV D. A. (2010). Long-term potentiation depends on release of D-serine from astrocytes. Nature, 463, 232-236.
HOSLI E. & HOSLI L. (1986). Binding sites for [3H]dopamine and dopamine-antagonists on cultured astrocytes of rat striatum and spinal cord: an autoradiographic study. Neurosci Lett, 65, 177-182.
HUANG C., WU J., LIAO R. & ZHANG W. (2012). SKF83959, an agonist of phosphatidylinositol-linked D(1)-like receptors, promotes ERK1/2 activation and cell migration in cultured rat astrocytes. PLoS One, 7, e49954.
INAZU M., KUBOTA N., TAKEDA H., ZHANG J., KIUCHI Y., OGUCHI K. & MATSUMIYA T. (1999a). Pharmacological characterization of dopamine transport in cultured rat astrocytes. Life Sci, 64, 2239-2245.
INAZU M., TAKEDA H., IKOSHI H., UCHIDA Y., KUBOTA N., KIUCHI Y., OGUCHI K. & MATSUMIYA T. (1999b). Regulation of dopamine uptake by basic fibroblast growth factor and epidermal growth factor in cultured rat astrocytes. Neurosci Res, 34, 235-244.
INAZU M., TAKEDA H. & MATSUMIYA T. (2003). Functional expression of the norepinephrine transporter in cultured rat astrocytes. J Neurochem, 84, 136-144.
IVERSEN L.L., I. S., DUNNETT S.B., BJORKLUND A. (2010). Dopamine Handbook. Oxford University Press.
JENNINGS A. (2014). Dopaminergic control of astrocytic calcium dynamics in situ and its potential effect on local synaptic activity. Doctoral thesis. UCL (University College London).
KEARN C. S., BLAKE-PALMER K., DANIEL E., MACKIE K. & GLASS M. (2005). Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol, 67, 1697-1704.
KHAN Z. U., KOULEN P., RUBINSTEIN M., GRANDY D. K. & GOLDMAN-RAKIC P. S. (2001). An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proc Natl Acad Sci U S A, 98, 1964-1969.
KIMELBERG H. K., CAI Z., RASTOGI P., CHARNIGA C. J., GODERIE S., DAVE V. & JALONEN T. O. (1997). Transmitter-induced calcium responses differ in astrocytes acutely isolated from rat brain and in culture. J Neurochem, 68, 1088-1098.
KITTEL-SCHNEIDER S., KENIS G., SCHEK J., VAN DEN HOVE D., PRICKAERTS J., LESCH K. P., STEINBUSCH H. & REIF A. (2012). Expression of monoamine transporters, nitric oxide synthase 3, and neurotrophin genes in antidepressant-stimulated astrocytes. Front Psychiatry, 3, 33.
KOEPSELL H., LIPS K. & VOLK C. (2007). Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res, 24, 1227-1251.
LEE S. P., SO C. H., RASHID A. J., VARGHESE G., CHENG R., LANCA A. J., O’DOWD B. F. & GEORGE S. R. (2004). Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem, 279, 35671-35678.
LEYTON M. & VEZINA P. (2014). Dopamine ups and downs in vulnerability to addictions: a neurodevelopmental model. Trends Pharmacol Sci, 35, 268-276.
LI A., GUO H., LUO X., SHENG J., YANG S., YIN Y., ZHOU J. & ZHOU J. (2006). Apomorphine-induced activation of dopamine receptors modulates FGF-2 expression in astrocytic cultures and promotes survival of dopaminergic neurons. Faseb j, 20, 1263-1265.
LI J., ZHU S., KOZONO D., NG K., FUTALAN D., SHEN Y., AKERS J. C., STEED T., KUSHWAHA D., SCHLABACH M., CARTER B. S., KWON C. H., FURNARI F., CAVENEE W., ELLEDGE S. & CHEN C. C. (2014). Genome-wide shRNA screen revealed integrated mitogenic signaling between dopamine receptor D2 (DRD2) and epidermal growth factor receptor (EGFR) in glioblastoma. Oncotarget, 5, 882-893.
LIU J., WANG F., HUANG C., LONG L. H., WU W. N., CAI F., WANG J. H., MA L. Q. & CHEN J. G. (2009). Activation of phosphatidylinositol-linked novel D1 dopamine receptor contributes to the calcium mobilization in cultured rat prefrontal cortical astrocytes. Cell Mol Neurobiol, 29, 317-328.
LORANG D., AMARA S. G. & SIMERLY R. B. (1994). Cell-type-specific expression of catecholamine transporters in the rat brain. J Neurosci, 14, 4903-4914.
LUO Y., KOKKONEN G. C., HATTORI A., CHREST F. J. & ROTH G. S. (1999). Dopamine stimulates redox-tyrosine kinase signaling and p38 MAPK in activation of astrocytic C6-D2L cells. Brain Res, 850, 21-38.
MARTINS-DE-SOUZA D., LEBAR M. & TURCK C. W. (2011). Proteome analyses of cultured astrocytes treated with MK-801 and clozapine: similarities with schizophrenia. Eur Arch Psychiatry Clin Neurosci, 261, 217-228.
MELTZER H. Y. & MCGURK S. R. (1999). The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull, 25, 233-255.
MING Y., ZHANG H., LONG L., WANG F., CHEN J. & ZHEN X. (2006). Modulation of Ca2+ signals by phosphatidylinositol-linked novel D1 dopamine receptor in hippocampal neurons. J Neurochem, 98, 1316-1323.
MIYAZAKI I., ASANUMA M., DIAZ-CORRALES F. J., MIYOSHI K. & OGAWA N. (2004). Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res, 1029, 120-123.
MONTERO T. D. & ORELLANA J. A. (2015). Hemichannels: new pathways for gliotransmitter release. Neuroscience, 286, 45-59.
NAGANUMA F., YOSHIKAWA T., NAKAMURA T., IIDA T., HARADA R., MOHSEN A. S., MIURA Y. & YANAI K. (2014). Predominant role of plasma membrane monoamine transporters in monoamine transport in 1321N1, a human astrocytoma-derived cell line. J Neurochem, 129, 591-601.
NARDIN P., TRAMONTINA A. C., QUINCOZES-SANTOS A., TORTORELLI L. S., LUNARDI P., KLEIN P. R., WARTCHOW K. M., BOBERMIN L. D., GOTTFRIED C., ELISABETSKY E. & GONCALVES C. A. (2011). In vitro S100B secretion is reduced by apomorphine: effects of antipsychotics and antioxidants. Prog Neuropsychopharmacol Biol Psychiatry, 35, 1291-1296.
NAVARRETE M. & ARAQUE A. (2010). Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron, 68, 113-126.
NUTT D. J., LINGFORD-HUGHES A., ERRITZOE D. & STOKES P. R. (2015). The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci, 16, 305-312.
OHTA K., KUNO S., INOUE S., IKEDA E., FUJINAMI A. & OHTA M. (2010). The effect of dopamine agonists: the expression of GDNF, NGF, and BDNF in cultured mouse astrocytes. J Neurol Sci, 291, 12-16.
ORDWAY GA S. M., FRAZER A. (2007). Cambridge University Press.
OTMAKHOVA N. A. & LISMAN J. E. (1999). Dopamine selectively inhibits the direct cortical pathway to the CA1 hippocampal region. J Neurosci, 19, 1437-1445.
PACHOLCZYK T., BLAKELY R. D. & AMARA S. G. (1991). Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature, 350, 350-354.
PARPURA V. & HAYDON P. G. (2000). Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proc Natl Acad Sci U S A, 97, 8629-8634.
PELTON E. W., 2ND KIMELBERG H. K., SHIPHERD S. V. & BOURKE R. S. (1981). Dopamine and norepinephrine uptake and metabolism by astroglial cells in culture. Life Sci, 28, 1655-1663.
PEREA G. & ARAQUE A. (2007). Astrocytes potentiate transmitter release at single hippocampal synapses. Science, 317, 1083-1086.
RASHID A. J., SO C. H., KONG M. M., FURTAK T., EL-GHUNDI M., CHENG R., O’DOWD B. F. & GEORGE S. R. (2007). D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A, 104, 654-659.
REQUARDT R. P., HIRRLINGER P. G., WILHELM F., WINKLER U., BESSER S. & HIRRLINGER J. (2012). Ca2+ signals of astrocytes are modulated by the NAD(+)/NADH redox state. J Neurochem, 120, 1014-1025.
REQUARDT R. P., WILHELM F., RILLICH J., WINKLER U. & HIRRLINGER J. (2010). The biphasic NAD(P)H fluorescence response of astrocytes to dopamine reflects the metabolic actions of oxidative phosphorylation and glycolysis. J Neurochem, 115, 483-492.
REUSS B. & UNSICKER K. (2001). Atypical neuroleptic drugs downregulate dopamine sensitivity in rat cortical and striatal astrocytes. Mol Cell Neurosci, 18, 197-209.
RUSAKOV D. A. (2015). Disentangling calcium-driven astrocyte physiology. Nat Rev Neurosci, 16, 226-233.
RUSAKOV D. A., BARD L., STEWART M. G. & HENNEBERGER C. (2014). Diversity of astroglial functions alludes to subcellular specialisation. Trends Neurosci, 37, 228-242.
RUSAKOV D. A., ZHENG K. & HENNEBERGER C. (2011). Astrocytes as regulators of synaptic function: a quest for the Ca2+ master key. Neuroscientist, 17, 513-523.
RUSS H., STAUST K., MARTEL F., GLIESE M. & SCHOMIG E. (1996). The extraneuronal transporter for monoamine transmitters exists in cells derived from human central nervous system glia. Eur J Neurosci, 8, 1256-1264.
SANTELLO M. & VOLTERRA A. (2012). TNFalpha in synaptic function: switching gears. Trends Neurosci, 35, 638-647.
SCHNIEDER T. P. & DWORK A. J. (2011). Searching for neuropathology: gliosis in schizophrenia. Biol Psychiatry, 69, 134-139.
SCHROETER S., APPARSUNDARAM S., WILEY R. G., MINER L. H., SESACK S. R. & BLAKELY R. D. (2000). Immunolocalization of the cocaine- and antidepressant-sensitive l-norepinephrine transporter. J Comp Neurol, 420, 211-232.
SHAO W., ZHANG S. Z., TANG M., ZHANG X. H., ZHOU Z., YIN Y. Q., ZHOU Q. B., HUANG Y. Y., LIU Y. J., WAWROUSEK E., CHEN T., LI S. B., XU M., ZHOU J. N., HU G. & ZHOU J. W. (2013). Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature, 494, 90-94.
SHAO Y. & MCCARTHY K. D. (1993). Regulation of astroglial responsiveness to neuroligands in primary culture. Neuroscience, 55, 991-1001.
SHAO Y. & MCCARTHY K. D. (1995). Receptor-mediated calcium signals in astroglia: multiple receptors, common stores and all-or-nothing responses. Cell Calcium, 17, 187-196.
SNYDER G. L., FIENBERG A. A., HUGANIR R. L. & GREENGARD P. (1998). A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci, 18, 10297-10303.
STEINER J., SCHROETER M. L., SCHILTZ K., BERNSTEIN H. G., MULLER U. J., RICHTER-LANDSBERG C., MULLER W. E., WALTER M., GOS T., BOGERTS B. & KEILHOFF G. (2010). Haloperidol and clozapine decrease S100B release from glial cells. Neuroscience, 167, 1025-1031.
SURMEIER D. J., BARGAS J., HEMMINGS H. C., JR., NAIRN A. C. & GREENGARD P. (1995). Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron, 14, 385-397.
TAKEDA H., INAZU M. & MATSUMIYA T. (2002). Astroglial dopamine transport is mediated by norepinephrine transporter. Naunyn Schmiedebergs Arch Pharmacol, 366, 620-623.
TANAHASHI S., YAMAMURA S., NAKAGAWA M., MOTOMURA E. & OKADA M. (2012). Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes. Br J Pharmacol, 165, 1543-1555.
TORRES G. E., GAINETDINOV R. R. & CARON M. G. (2003). Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci, 4, 13-25.
TORVINEN M., KOZELL L. B., NEVE K. A., AGNATI L. F. & FUXE K. (2004). Biochemical identification of the dopamine D2 receptor domains interacting with the adenosine A2A receptor. J Mol Neurosci, 24, 173-180.
TORVINEN M., MARCELLINO D., CANALS M., AGNATI L. F., LLUIS C., FRANCO R. & FUXE K. (2005). Adenosine A2A receptor and dopamine D3 receptor interactions: evidence of functional A2A/D3 heteromeric complexes. Mol Pharmacol, 67, 400-407.
VAARMANN A., GANDHI S. & ABRAMOV A. Y. (2010). Dopamine induces Ca2+ signaling in astrocytes through reactive oxygen species generated by monoamine oxidase. J Biol Chem, 285, 25018-25023.
VAUGHAN R. A. & FOSTER J. D. (2013). Mechanisms of dopamine transporter regulation in normal and disease states. Trends Pharmacol Sci, 34, 489-496.
VERKHRATSKY A, B. A. (2007). Glial Neurobiology: A Textbook: Wiley.
VERMEULEN R. J., JONGENELEN C. A., LANGEVELD C. H., WOLTERS E. C., STOOF J. C. & DRUKARCH B. (1994). Dopamine D1 receptor agonists display a different intrinsic activity in rat, monkey and human astrocytes. Eur J Pharmacol, 269, 121-125.
VOLLBRECHT P. J., SIMMLER L. D., BLAKELY R. D. & DEUTCH A. Y. (2014). Dopamine denervation of the prefrontal cortex increases expression of the astrocytic glutamate transporter GLT-1. J Neurochem, 130, 109-114.
VOLTERRA A., LIAUDET N. & SAVTCHOUK I. (2014). Astrocyte Ca2+ signalling: an unexpected complexity. Nat Rev Neurosci, 15, 327-335.
YOUDIM M. B. & BAKHLE Y. S. (2006). Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. Br J Pharmacol, 147 Suppl 1, S287-296.
ZANASSI P., PAOLILLO M., MONTECUCCO A., AVVEDIMENTO E. V. & SCHINELLI S. (1999). Pharmacological and molecular evidence for dopamine D(1) receptor expression by striatal astrocytes in culture. J Neurosci Res, 58, 544-552.