In this study we developed microfluidic structure with two neuronal cultures grown in separated chambers and connected by microchannels for axon outgrowth. We estimated bursting activity transfer characteristics between chambers in relation toculture development and determine rate of axonal growth in chambers.
INTRODUCTION
Microfluidics and microelectrode technology recently have been advanced in neuroengeneering in fundamental research of the brain and medical applications. Neuronal cells grown in microfluidic structures allow to isolate the cell populations in individual compartments connected by microchannels in which axons and dendrites can grow. The cultivation of neural networks on the microelectrode arrays using multichannel electrophysiology system makes possible to observe development and functional state of connections, their enhancement or suppression.
RESULTS
In this study we designed a microfluidic device combined with microelectrode arraywhich allows to grow two separate cultured neuronal networks with directed synaptic pathways inbetween. Microchannels’ length was 400 μm and 600 μm.We found that 4 days was enough for axons to growthrough the whole channel from presynaptic chamber (chamber A) to postsynaptic chamber (chamber B) (Fig.1).
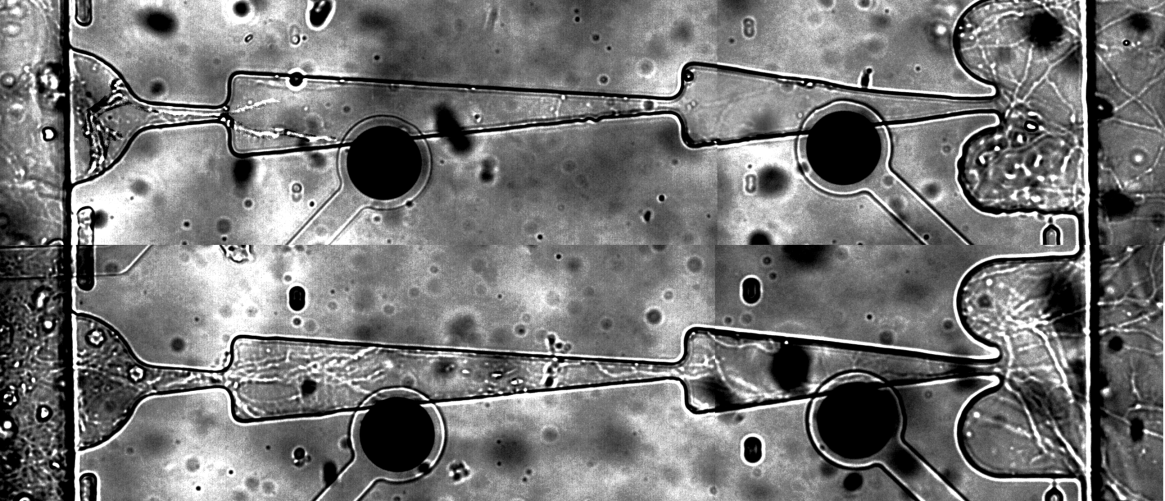
Fig. 1.Axons outgrowth in microchannels on day 2(a) and day 4(b).
The averagevelocity of axonal growth in microchannel was found to be13.8 μm per hour. Next we investigated spontaneous activity propagationthrough axonal pathways from one chamber to another during 30 days ofculture development. Specific design of the microchannels defined axon outgrowth. Percent of burst’s transmitting in intended direction was changed depending on number of burst evoked in neuronal culture and increasedduring maturation (Fig. 2, c). Percent of the burst’s propagation in opposite direction decreasedduring development (Fig. 2, d) and the ratio of directed against backward propagation increased (Fig. 2, e). We suppose that with culture development strength of synaptic connection increased and the activity propagated primary inintended direction.
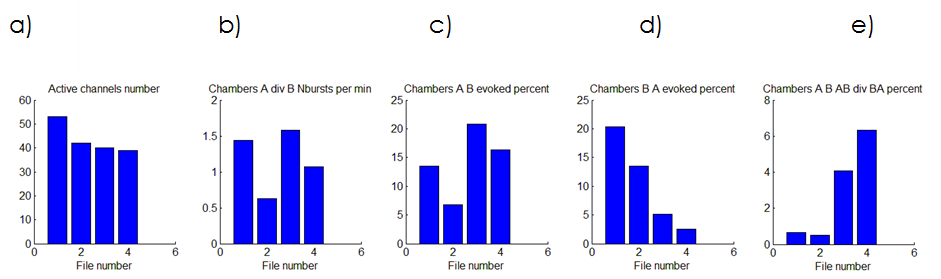
Fig. 2.Change of spontaneous signal propagation during culture development (15, 20, 25 , 30 days in vitro).The number of active electrodes (a), ratio of spontaneous bursting frequency recorded in two chambers (b), percent of bursts evoked from chamber A to chamber B (c), from chamber B to chamber A (d) and their relation (d).
| Attachment | Size |
|---|---|
| 542.87 KB |
This research was supported by Russian Science Foundation (№ 14-19-01381).
Liangbin P., Sankaraleengam A., Eric F., Stathis S. L., Thomas B. D., Gregory J. B. and Bruce C. W. (2015). An in vitro method to manipulate the direction and functional strength between neural populations. Frontiers in Neural Circuits, 14(July).
Le Feber J., Postma W., Rutten W., De Weerd E.,Weusthof M.. Two-Chamber MEA to Unidirectionally Connect Neuronal Cultures. Proceeding of 9th Int. Meeting on Substrate-Integrated Microelectrode Arrays, 2014. pp. 259-262.


