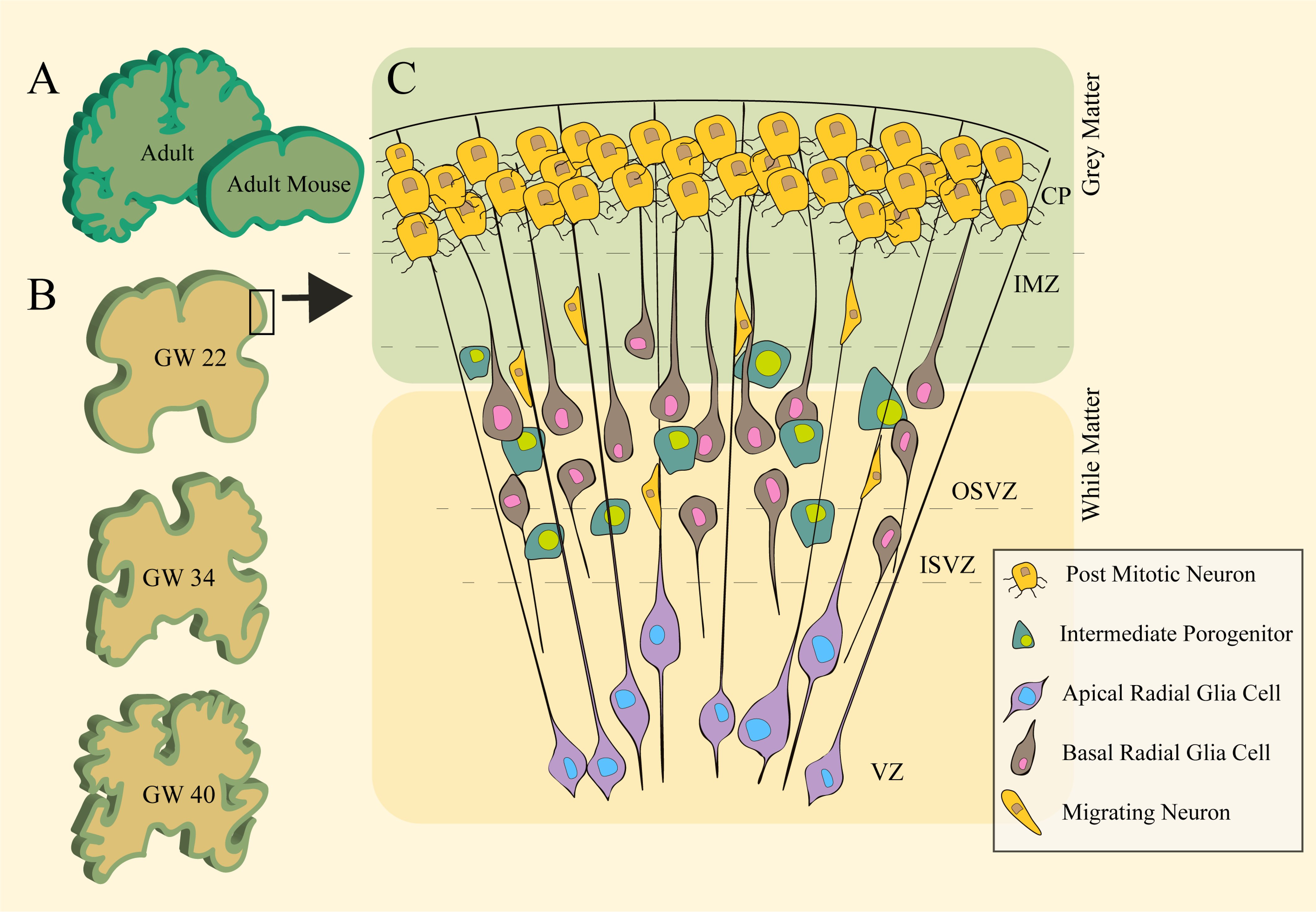
The convoluted human cerebral cortex is one of the key features that allows for an increased neuronal density packing essential for the complex cognitive and socioemotional behaviours man possesses. Nevertheless, the underlying mechanisms involved in cortical folding remained a both intriguing and functionally important enigma. A crucial component known to be involved in the formation and maintenance of all tissues is the extracellular matrix (ECM), providing scaffolds which tie tissues and organs in place. The composition of the ECM in both developing and mature structures is constantly remodelled, degraded and secreted by numerous types of cells, and its role as a source of growth factors and signalling in morphogenesis, migration, and proliferation is increasingly appreciated. Evidence for the differential expression of ECM during gyrification pinpoints its potentially fundamental role in shaping the folds of the cerebral cortex through both mechanical and molecular configurations. This review aims at addressing key ideas, potential directions and discoveries that highlight biomechanics of the ECM during the construction of the cortex cerebral gyrification.
| Attachment | Size |
|---|---|
| 1.26 MB |
We would like to acknowledge current and previous lab members for their helpful discussions, and to thank Moran Keren Zur for his assistance in preparing the figures for this article.
Our research has been supported (to O.R.) in part by the Israel Science Foundation (Grant No. 347/15) and the Legacy Heritage Biomedical Program of the Israel Science Foundation (Grant No. 2041/16), ISF-NSFC joint research program (Grant No. 2449/16), this work was carried out with the aid of a grant no. 2397/18 from the Canadian Institutes of Health Research (CIHR), the International Development Research Centre (IDRC), the Israel Science Foundation (ISF), ERA-NET Neuron with support of the IMOH (Grant No. 3-0000-12276), German-Israeli Foundation (GIF) (Grant no. I-1476-203.13/2018), United States -Israel Binational Science Foundation (BSF) (Grant no. 2017006), Nella and Leon Benoziyo Center for Neurological Diseases, Jeanne and Joseph Nissim Foundation for Life Sciences Research, Wohl Biology Endowment Fund, Lulu P. and David J. Levidow Fund for Alzheimer’s Diseases and Neuroscience Research, the Helen and Martin Kimmel Stem Cell Research Institute, the Kekst Family Institute for Medical Genetics, the David and Fela Shapell Family Center for Genetic Disorders Research. O.R. is an Incumbent of the Berstein-Mason professorial chair of Neurochemistry.
Acharya, C., Yik, J. H., Kishore, A., Van Dinh, V., Di Cesare, P. E., & Haudenschild, D. R. (2014). Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol, 37, 102-111.
Bangasser, B. L., Shamsan, G. A., Chan, C. E., Opoku, K. N., Tüzel, E., Schlichtmann, B. W., . . . Odde, D. J. (2017). Shifting the optimal stiffness for cell migration. Nat Commun, 8, 15313.
Barkovich, A. J., Guerrini, R., Kuzniecky, R. I., Jackson, G. D., & Dobyns, W. B. (2012). A developmental and genetic classification for malformations of cortical development: update 2012. Brain, 135, 1348-1369.
Barkovich, A. J., Jackson, D. E., Jr., & Boyer, R. S. (1989). Band heterotopias: a newly recognized neuronal migration anomaly. Radiology, 171, 455-458.
Barkovich, A. J., Kuzniecky, R. I., Jackson, G. D., Guerrini, R., & Dobyns, W. B. (2005). A developmental and genetic classification for malformations of cortical development. Neurology, 65, 1873-1887.
Barros, C. S., Franco, S. J., & Muller, U. (2011). Extracellular matrix: functions in the nervous system. Cold Spring Harb Perspect Biol, 3, a005108.
Bowden, N., Brittain, S., Evans, A. G., Hutchinson, J. W., & Whitesides, G. M. (1998). Spontaneous formation of ordered structures in thin films of metals supported on an elastomeric polymer. Nature, 393, 146-149.
Cerda, E., & Mahadevan, L. (2003). Geometry and physics of wrinkling. Phys Rev Lett, 90, 074302.
Chenn, A., & Walsh, C. A. (2002). Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science, 297, 365-369.
Dehay, C., Kennedy, H., & Kosik, Kenneth S. (2015). The Outer Subventricular Zone and Primate-Specific Cortical Complexification. Neuron, 85, 683-694.
del Toro, D., Ruff, T., Cederfjäll, E., Villalba, A., Seyit-Bremer, G., Borrell, V., & Klein, R. (2017). Regulation of Cerebral Cortex Folding by Controlling Neuronal Migration via FLRT Adhesion Molecules. Cell, 169, 621-635.e616.
Dervaux, J., Couder, Y., Guedeau-Boudeville, M. A., & Ben Amar, M. (2011). Shape transition in artificial tumors: from smooth buckles to singular creases. Phys Rev Lett, 107, 018103.
Dityatev, A., Schachner, M., & Sonderegger, P. (2010). The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci, 11, 735-746.
Dobyns, W. B. (2010). The clinical patterns and molecular genetics of lissencephaly and subcortical band heterotopia. Epilepsia, 51, 5-9.
Engler, A. J., Sen, S., Sweeney, H. L., & Discher, D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell, 126, 677-689.
Fernandez, V., Llinares-Benadero, C., & Borrell, V. (2016). Cerebral cortex expansion and folding: what have we learned? EMBO J, 35, 1021-1044.
Fernández, V., Llinares‐Benadero, C., & Borrell, V. (2016). Cerebral cortex expansion and folding: what have we learned? Embo j, 35, 1021.
Fietz, S. A., Kelava, I., Vogt, J., Wilsch-Bräuninger, M., Stenzel, D., Fish, J. L., . . . Huttner, W. B. (2010). OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci, 13, 690.
Fietz, S. A., Lachmann, R., Brandl, H., Kircher, M., Samusik, N., Schroder, R., . . . Huttner, W. B. (2012). Transcriptomes of germinal zones of human and mouse fetal neocortex suggest a role of extracellular matrix in progenitor self-renewal. Proc Natl Acad Sci U S A, 109, 11836-11841.
Florio, M., Albert, M., Taverna, E., Namba, T., Brandl, H., Lewitus, E., . . . Huttner, W. B. (2015). Human-specific gene <em>ARHGAP11B</em> promotes basal progenitor amplification and neocortex expansion. Science, 347, 1465.
Florio, M., Borrell, V., & Huttner, W. B. (2017). Human-specific genomic signatures of neocortical expansion. Curr Opin Neurobiol, 42, 33-44.
Florio, M., & Huttner, W. B. (2014). Neural progenitors, neurogenesis and the evolution of the neocortex. Development, 141, 2182.
Folsom, T. D., & Fatemi, S. H. (2013). The involvement of Reelin in neurodevelopmental disorders. Neuropharmacology, 68, 122-135.
Fowke, T. M., Karunasinghe, R. N., Bai, J.-Z., Jordan, S., Gunn, A. J., & Dean, J. M. (2017). Hyaluronan synthesis by developing cortical neurons in vitro. Scientific Reports, 7, 44135.
Fraser, J. R. E., Laurent, T. C., & Laurent, U. B. G. (1997). Hyaluronan: its nature, distribution, functions and turnover. Journal of Internal Medicine, 242, 27-33.
Gaiano, N., Nye, J. S., & Fishell, G. (2000). Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron, 26, 395-404.
Georges-Labouesse, E., Mark, M., Messaddeq, N., & Gansmuller, A. (1998). Essential role of alpha 6 integrins in cortical and retinal lamination. Curr Biol, 8, 983-986.
Gotz, M., & Huttner, W. B. (2005). The cell biology of neurogenesis. Nat Rev Mol Cell Biol, 6, 777-788.
Groenewold, J. (2001). Wrinkling of plates coupled with soft elastic media. Physica A, 298, 32-45.
Hansen, D. V., Rubenstein, J. L., & Kriegstein, A. R. (2011). Deriving excitatory neurons of the neocortex from pluripotent stem cells. Neuron, 70, 645-660.
Haque, M. A., Nagaoka, M., Hexig, B., & Akaike, T. (2010). Artificial extracellular matrix for embryonic stem cell cultures: a new frontier of nanobiomaterials. Sci Technol Adv Mater, 11, 014106.
Holle, A. W., Young, J. L., Van Vliet, K. J., Kamm, R. D., Discher, D., Janmey, P., . . . Saif, T. (2018). Cell-Extracellular Matrix Mechanobiology: Forceful Tools and Emerging Needs for Basic and Translational Research. Nano Lett, 18, 1-8.
Honda, T., Kobayashi, K., Mikoshiba, K., & Nakajima, K. (2011). Regulation of cortical neuron migration by the Reelin signaling pathway. Neurochem Res, 36, 1270-1279.
Hong, S. E., Shugart, Y. Y., Huang, D. T., Shahwan, S. A., Grant, P. E., Hourihane, J. O., Walsh, C. A. (2000). Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat Genet, 26, 93-96.
Hummel, V., Kallmann, B. A., Wagner, S., Füller, T., Bayas, A., Tonn, J. C., . . . Rieckmann, P. (2001). Production of MMPs in Human Cerebral Endothelial Cells and Their Role in Shedding Adhesion Molecules. Journal of Neuropathology & Experimental Neurology, 60, 320-327.
Karzbrun, E., Kshirsagar, A., Cohen, S. R., Hanna, J. H., & Reiner, O. (2018). Human brain organoids on a chip reveal the physics of folding. Nature Physics.
Kim, Y., Ko, H., Kwon, I. K., & Shin, K. (2016). Extracellular Matrix Revisited: Roles in Tissue Engineering. Int Neurourol J, 20, S23-29.
Kuida, K., Haydar, T. F., Kuan, C. Y., Gu, Y., Taya, C., Karasuyama, H., . . . Flavell, R. A. (1998). Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell, 94, 325-337.
Lewitus, E., Kelava, I., & Huttner, W. B. (2013). Conical expansion of the outer subventricular zone and the role of neocortical folding in evolution and development. Frontiers in Human Neuroscience, 7, 424.
Li, Y., Muffat, J., Omer, A., Bosch, I., Lancaster, M. A., Sur, M., Jaenisch, R. (2017a). Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell, 20, 385-396.e383.
Li, Y., Muffat, J., Omer, A., Bosch, I., Lancaster, M. A., Sur, M., . . . Jaenisch, R. (2017b). Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell, 20, 385-396 e383.
Long, K. R., Newland, B., Florio, M., Kalebic, N., Langen, B., Kolterer, A., . . . Huttner, W. B. (2018). Extracellular Matrix Components HAPLN1, Lumican, and Collagen I Cause Hyaluronic Acid-Dependent Folding of the Developing Human Neocortex. Neuron, 99, 702-719.e707.
Lu, P., Takai, K., Weaver, V. M., & Werb, Z. (2011). Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol, 3.
Lui, Jan H., Hansen, David V., & Kriegstein, Arnold R. (2011). Development and Evolution of the Human Neocortex. Cell, 146, 18-36.
Martynoga, B., Drechsel, D., & Guillemot, F. (2012). Molecular control of neurogenesis: a view from the mammalian cerebral cortex. Cold Spring Harb Perspect Biol, 4.
Miron-Mendoza, M., Seemann, J., & Grinnell, F. (2010). The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials, 31, 6425-6435.
Miyata, S., & Kitagawa, H. (2017). Formation and remodeling of the brain extracellular matrix in neural plasticity: Roles of chondroitin sulfate and hyaluronan. Biochim Biophys Acta, 1861, 2420-2434.
Nonaka-Kinoshita, M., Reillo, I., Artegiani, B., Martinez-Martinez, M. A., Nelson, M., Borrell, V., & Calegari, F. (2013). Regulation of cerebral cortex size and folding by expansion of basal progenitors. Embo j, 32, 1817-1828.
Olson, E. C., & Walsh, C. A. (2002). Smooth, rough and upside-down neocortical development. Current Opinion in Genetics & Development, 12, 320-327.
Pollen, Alex A., Nowakowski, Tomasz J., Chen, J., Retallack, H., Sandoval-Espinosa, C., Nicholas, Cory R., . . . Kriegstein, Arnold R. (2015). Molecular Identity of Human Outer Radial Glia during Cortical Development. Cell, 163, 55-67.
Raab, M., & Hancock, W. O. (2008). Transport and detection of unlabeled nucleotide targets by microtubules functionalized with molecular beacons. Biotechnology and Bioengineering, 99, 764-773.
Rauch, U. (2004). Extracellular matrix components associated with remodeling processes in brain. Cell Mol Life Sci, 61, 2031-2045.
Reillo, I., de Juan Romero, C., García-Cabezas, M. Á., & Borrell, V. (2011). A Role for Intermediate Radial Glia in the Tangential Expansion of the Mammalian Cerebral Cortex. Cerebral Cortex, 21, 1674-1694.
Reiner, O., & Sapir, T. (2005). Similarities and differences between the Wnt and reelin pathways in the forming brain. Mol Neurobiol, 31, 117-134.
Reiner, O., & Sapir, T. (2013). LIS1 functions in normal development and disease. Curr Opin Neurobiol, 23, 951-956.
Schmid, R. S., & Anton, E. S. (2003). Role of Integrins in the Development of the Cerebral Cortex. Cerebral Cortex, 13, 219-224.
Schwartz, M. A. (2010). Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol, 2, a005066.
Schweitzer, B., Singh, J., Fejtova, A., Groc, L., Heine, M., & Frischknecht, R. (2017). Hyaluronic acid based extracellular matrix regulates surface expression of GluN2B containing NMDA receptors. Scientific Reports, 7, 10991.
Sekine, K., Kubo, K., & Nakajima, K. (2014). How does Reelin control neuronal migration and layer formation in the developing mammalian neocortex? Neurosci Res, 86, 50-58.
Solis, M. A., Chen, Y.-H., Wong, T. Y., Bittencourt, V. Z., Lin, Y.-C., & Huang, L. L. H. (2012). Hyaluronan Regulates Cell Behavior: A Potential Niche Matrix for Stem Cells. Biochemistry Research International, 2012, 346972.
Stahl, R., Walcher, T., De Juan Romero, C., Pilz, Gregor A., Cappello, S., Irmler, M., . . . Götz, M. (2013). Trnp1 Regulates Expansion and Folding of the Mammalian Cerebral Cortex by Control of Radial Glial Fate. Cell, 153, 535-549.
Tallinen, T., Chung, J. Y., Biggins, J. S., & Mahadevan, L. (2014a). Gyrification from constrained cortical expansion. Proceedings of the National Academy of Sciences, 111, 12667.
Tallinen, T., Chung, J. Y., Biggins, J. S., & Mahadevan, L. (2014b). Gyrification from constrained cortical expansion. Proc Natl Acad Sci U S A, 111, 12667-12672.
Tallinen, T., Chung, J. Y., Rousseau, F., Girard, N., Lefevre, J., & Mahadevan, L. (2016). On the growth and form of cortical convolutions. Nature Physics, 12, 588-593.
Tanaka, T., Sun, S. T., Hirokawa, Y., Katayama, S., Kucera, J., Hirose, Y., & Amiya, T. (1987). Mechanical Instability of Gels at the Phase-Transition. Nature, 325, 796-798.
Toole, B. P. (2004). Hyaluronan: from extracellular glue to pericellular cue. Nature Reviews Cancer, 4, 528.
van 't Spijker, H. M., & Kwok, J. C. F. (2017). A Sweet Talk: The Molecular Systems of Perineuronal Nets in Controlling Neuronal Communication. Frontiers in Integrative Neuroscience, 11.